Human neutrophils phagocytose and kill Acinetobacter baumannii and A. pittii
- PMID: 28676640
- PMCID: PMC5496873
- DOI: 10.1038/s41598-017-04870-8
Human neutrophils phagocytose and kill Acinetobacter baumannii and A. pittii
Erratum in
-
Author Correction: Human neutrophils phagocytose and kill Acinetobacter baumannii and A. pittii.Sci Rep. 2020 Mar 11;10(1):4797. doi: 10.1038/s41598-020-61184-y. Sci Rep. 2020. PMID: 32161313 Free PMC article.
Abstract
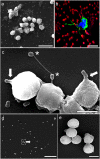
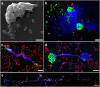
Acinetobacter baumannii is a common cause of health care associated infections worldwide. A. pittii is an opportunistic pathogen also frequently isolated from Acinetobacter infections other than those from A. baumannii. Knowledge of Acinetobacter virulence factors and their role in pathogenesis is scarce. Also, there are no detailed published reports on the interactions between A. pittii and human phagocytic cells. Using confocal laser and scanning electron microscopy, immunofluorescence, and live-cell imaging, our study shows that immediately after bacteria-cell contact, neutrophils rapidly and continuously engulf and kill bacteria during at least 4 hours of infection in vitro. After 3 h of infection, neutrophils start to release neutrophil extracellular traps (NETs) against Acinetobacter. DNA in NETs colocalizes well with human histone H3 and with the specific neutrophil elastase. We have observed that human neutrophils use large filopodia as cellular tentacles to sense local environment but also to detect and retain bacteria during phagocytosis. Furthermore, co-cultivation of neutrophils with human differentiated macrophages before infections shows that human neutrophils, but not macrophages, are key immune cells to control Acinetobacter. Although macrophages were largely activated by both bacterial species, they lack the phagocytic activity demonstrated by neutrophils.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Lahmer T, et al. Acinetobacter baumannii sepsis is fatal in medical intensive care unit patients: six cases and review of literature. Anaesth Intensive Care. 2014;42:666–668. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

