The multifaceted roles of PARP1 in DNA repair and chromatin remodelling
- PMID: 28676700
- PMCID: PMC6591728
- DOI: 10.1038/nrm.2017.53
The multifaceted roles of PARP1 in DNA repair and chromatin remodelling
Abstract
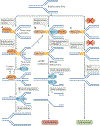
Cells are exposed to various endogenous and exogenous insults that induce DNA damage, which, if unrepaired, impairs genome integrity and leads to the development of various diseases, including cancer. Recent evidence has implicated poly(ADP-ribose) polymerase 1 (PARP1) in various DNA repair pathways and in the maintenance of genomic stability. The inhibition of PARP1 is therefore being exploited clinically for the treatment of various cancers, which include DNA repair-deficient ovarian, breast and prostate cancers. Understanding the role of PARP1 in maintaining genome integrity is not only important for the design of novel chemotherapeutic agents, but is also crucial for gaining insights into the mechanisms of chemoresistance in cancer cells. In this Review, we discuss the roles of PARP1 in mediating various aspects of DNA metabolism, such as single-strand break repair, nucleotide excision repair, double-strand break repair and the stabilization of replication forks, and in modulating chromatin structure.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures




References
-
- Lord CJ, Tutt AN & Ashworth A Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu. Rev. Med 66, 455–470 (2015). - PubMed
-
- Ame JC, Spenlehauer C & de Murcia G The PARP superfamily. Bioessays 26, 882–893 (2004). - PubMed
-
- Buki KG & Kun E Polypeptide domains of ADP-ribosyltransferase obtained by digestion with plasmin. Biochemistry 27, 5990–5995 (1988). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

