A channelopathy mutation in the voltage-sensor discloses contributions of a conserved phenylalanine to gating properties of Kv1.1 channels and ataxia
- PMID: 28676720
- PMCID: PMC5496848
- DOI: 10.1038/s41598-017-03041-z
A channelopathy mutation in the voltage-sensor discloses contributions of a conserved phenylalanine to gating properties of Kv1.1 channels and ataxia
Abstract
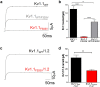
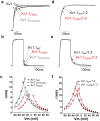
Channelopathy mutations prove informative on disease causing mechanisms and channel gating dynamics. We have identified a novel heterozygous mutation in the KCNA1 gene of a young proband displaying typical signs and symptoms of Episodic Ataxia type 1 (EA1). This mutation is in the S4 helix of the voltage-sensing domain and results in the substitution of the highly conserved phenylalanine 303 by valine (p.F303V). The contributions of F303 towards K+ channel voltage gating are unclear and here have been assessed biophysically and by performing structural analysis using rat Kv1.2 coordinates. We observed significant positive shifts of voltage-dependence, changes in the activation, deactivation and slow inactivation kinetics, reduced window currents, and decreased current amplitudes of both Kv1.1 and Kv1.1/1.2 channels. Structural analysis revealed altered interactions between F303V and L339 and I335 of the S5 helix of a neighboring subunit. The substitution of an aromatic phenylalanine with an aliphatic valine within the voltage-sensor destabilizes the open state of the channel. Thus, F303 fine-tunes the Kv1.1 gating properties and contributes to the interactions between the S4 segment and neighboring alpha helices. The resulting channel's loss of function validates the clinical relevance of the mutation for EA1 pathogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Comment in
-
Commentary: A channelopathy mutation in the voltage-sensor discloses contributions of a conserved phenylalanine to gating properties of Kv1.1 channels and ataxia.Front Cell Neurosci. 2018 Jun 20;12:174. doi: 10.3389/fncel.2018.00174. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29973872 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

