Overexpression of a Grapevine Sucrose Transporter (VvSUC27) in Tobacco Improves Plant Growth Rate in the Presence of Sucrose In vitro
- PMID: 28676814
- PMCID: PMC5476780
- DOI: 10.3389/fpls.2017.01069
Overexpression of a Grapevine Sucrose Transporter (VvSUC27) in Tobacco Improves Plant Growth Rate in the Presence of Sucrose In vitro
Erratum in
-
Corrigendum: Overexpression of a Grapevine Sucrose Transporter (VvSUC27) in Tobacco Improves Plant Growth Rate in the Presence of Sucrose In vitro.Front Plant Sci. 2017 Oct 16;8:1817. doi: 10.3389/fpls.2017.01817. eCollection 2017. Front Plant Sci. 2017. PMID: 29056943 Free PMC article.
-
Corrigendum: Overexpression of a Grapevine Sucrose Transporter (VvSUC27) in Tobacco Improves Plant Growth Rate in the Presence of Sucrose In vitro.Front Plant Sci. 2018 Feb 5;9:36. doi: 10.3389/fpls.2018.00036. eCollection 2018. Front Plant Sci. 2018. PMID: 29434614 Free PMC article.
Abstract
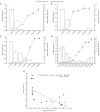
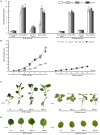
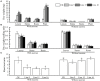
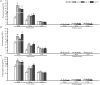
The import of sugar from source leaves and it further accumulation in grape berries are considerably high during ripening, and this process is mediated via sucrose transporters. In this study, a grape sucrose transporter (SUT) gene, VvSUC27, located at the plasma membrane, was transferred to tobacco (Nicotiana tabacum). The transformants were more sensitive to sucrose and showed more rapid development, especially roots, when cultured on MS agar medium containing sucrose, considering that the shoot/root dry weight ratio was only half that of the control. Moreover, all transformed plants exhibited light-colored leaves throughout their development, which indicated chlorosis and an associated reduction in photosynthesis. The total sugar content in the roots and stems of transformants was higher than that in control plants. No significant difference was observed in the leaves between the transformants and control plants. The levels of growth-promoting hormones were increased, and those of stress-mediating hormones were reduced in transgenic tobacco plants. The qRT-PCR analysis revealed that the expression of VvSUC27 was 1,000 times higher than that of the autologous tobacco sucrose transporter, which suggested that the markedly increased growth rate of transformants was because of the heterogeneously expressed gene. The transgenic tobacco plants showed resistance to abiotic stresses. Strikingly, the overexpression of VvSUC27 leaded to the up regulation of most reactive oxygen species scavengers and abscisic acid-related genes that might enable transgenic plants to overcome abiotic stress. Taken together, these results revealed an important role of VvSUC27 in plant growth and response to abiotic stresses, especially in the presence of sucrose in vitro.
Keywords: VvSUC27; abiotic stresses; grapevine; growth; sucrose.
Figures








Similar articles
-
Corrigendum: Overexpression of a Grapevine Sucrose Transporter (VvSUC27) in Tobacco Improves Plant Growth Rate in the Presence of Sucrose In vitro.Front Plant Sci. 2018 Feb 5;9:36. doi: 10.3389/fpls.2018.00036. eCollection 2018. Front Plant Sci. 2018. PMID: 29434614 Free PMC article.
-
Corrigendum: Overexpression of a Grapevine Sucrose Transporter (VvSUC27) in Tobacco Improves Plant Growth Rate in the Presence of Sucrose In vitro.Front Plant Sci. 2017 Oct 16;8:1817. doi: 10.3389/fpls.2017.01817. eCollection 2017. Front Plant Sci. 2017. PMID: 29056943 Free PMC article.
-
Expression of Sucrose Transporters from Vitis vinifera Confer High Yield and Enhances Drought Resistance in Arabidopsis.Int J Mol Sci. 2020 Apr 9;21(7):2624. doi: 10.3390/ijms21072624. Int J Mol Sci. 2020. PMID: 32283825 Free PMC article.
-
ABA and GA3 increase carbon allocation in different organs of grapevine plants by inducing accumulation of non-structural carbohydrates in leaves, enhancement of phloem area and expression of sugar transporters.Physiol Plant. 2016 Mar;156(3):323-37. doi: 10.1111/ppl.12390. Epub 2015 Oct 26. Physiol Plant. 2016. PMID: 26411544
-
Role of magnesium in carbon partitioning and alleviating photooxidative damage.Physiol Plant. 2008 Aug;133(4):692-704. doi: 10.1111/j.1399-3054.2007.01042.x. Physiol Plant. 2008. PMID: 18724409 Review.
Cited by
-
QTL mapping and transcriptome analysis of sugar content during fruit ripening of Pyrus pyrifolia.Front Plant Sci. 2023 Mar 6;14:1137104. doi: 10.3389/fpls.2023.1137104. eCollection 2023. Front Plant Sci. 2023. PMID: 36950356 Free PMC article.
-
Hetero/Homo-Complexes of Sucrose Transporters May Be a Subtle Mode to Regulate Sucrose Transportation in Grape Berries.Int J Mol Sci. 2021 Nov 8;22(21):12062. doi: 10.3390/ijms222112062. Int J Mol Sci. 2021. PMID: 34769493 Free PMC article.
-
The transcription factors ERF105 and NAC72 regulate expression of a sugar transporter gene and hexose accumulation in grape.Plant Cell. 2024 Dec 23;37(1):koae326. doi: 10.1093/plcell/koae326. Plant Cell. 2024. PMID: 39691057
-
Ectopic expression of a grapevine alkaline α-galactosidase seed imbibition protein VvSIP enhanced salinity tolerance in transgenic tobacco plants.Funct Integr Genomics. 2022 Dec 22;23(1):12. doi: 10.1007/s10142-022-00945-6. Funct Integr Genomics. 2022. PMID: 36547729
-
Carbon and nitrogen metabolism affects kentucky bluegrass rhizome expansion.BMC Plant Biol. 2023 Apr 26;23(1):221. doi: 10.1186/s12870-023-04230-x. BMC Plant Biol. 2023. PMID: 37101108 Free PMC article.
References
-
- Ageorges A., Issaly R., Picaud S., Delrot S., Romieu C. (2000). Identification and functional expression in yeast of a grape berry sucrose carrier. Plant Physiol. Biochem. 38, 177–185. 10.1016/S0981-9428(00)00730-0 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

