Arabinogalactan Proteins Are Involved in Salt-Adaptation and Vesicle Trafficking in Tobacco by-2 Cell Cultures
- PMID: 28676820
- PMCID: PMC5476920
- DOI: 10.3389/fpls.2017.01092
Arabinogalactan Proteins Are Involved in Salt-Adaptation and Vesicle Trafficking in Tobacco by-2 Cell Cultures
Abstract
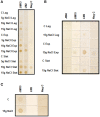
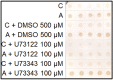
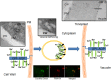
Arabinogalactan proteins (AGPs) are a highly diverse family of glycoproteins that are commonly found in most plant species. However, little is known about the physiological and molecular mechanisms of their function. AGPs are involved in different biological processes such as cell differentiation, cell expansion, tissue development and somatic embryogenesis. AGPs are also involved in abiotic stress response such as salinity modulating cell wall expansion. In this study, we describe how salt-adaptation in tobacco BY-2 cell cultures induces important changes in arabinogalactan proteins distribution and contents. Using the immuno-dot blot technique with different anti-AGP antibodies (JIM13, JIM15, and others), we observed that AGPs were highly accumulated in the culture medium of salt-adapted tobacco cells, probably due to the action of phospholipases. We located these AGP epitopes using immunogold labeling in the cytoplasm associated to the endoplasmic reticulum, the golgi apparatus, and vesicles, plasma membrane and tonoplast. Our results show that salt-adaptation induced a significant reduction of the cytoplasm, plasma membrane and tonoplast content of these epitopes. Yariv reagent was added to the control and salt-adapted tobacco cell cultures, leading to cell death induction in control cells but not in salt-adapted cells. Ultrastructural and immunogold labeling revealed that cell death induced by Yariv reagent in control cells was due to the interaction of Yariv reagent with the AGPs linked to the plasma membranes. Finally, we propose a new function of AGPs as a possible sodium carrier through the mechanism of vesicle trafficking from the apoplast to the vacuoles in salt-adapted tobacco BY-2 cells. This mechanism may contribute to sodium homeostasis during salt-adaptation to high saline concentrations.
Keywords: arabinogalactan proteins; phospholipase C; salinity adaptation; vesicle trafficking; yariv reagent.
Figures






Similar articles
-
Salt stress upregulates periplasmic arabinogalactan proteins: using salt stress to analyse AGP function.New Phytol. 2006;169(3):479-92. doi: 10.1111/j.1469-8137.2005.01591.x. New Phytol. 2006. PMID: 16411951
-
Multiflagellated sperm cells of Ceratopteris richardii are bathed in arabinogalactan proteins throughout development.Am J Bot. 2014 Dec;101(12):2052-61. doi: 10.3732/ajb.1400424. Epub 2014 Nov 16. Am J Bot. 2014. PMID: 25480702
-
Pectin De-methylesterification and AGP Increase Promote Cell Wall Remodeling and Are Required During Somatic Embryogenesis of Quercus suber.Front Plant Sci. 2019 Jan 8;9:1915. doi: 10.3389/fpls.2018.01915. eCollection 2018. Front Plant Sci. 2019. PMID: 30671070 Free PMC article.
-
Three Decades of Advances in Arabinogalactan-Protein Biosynthesis.Front Plant Sci. 2020 Dec 15;11:610377. doi: 10.3389/fpls.2020.610377. eCollection 2020. Front Plant Sci. 2020. PMID: 33384708 Free PMC article. Review.
-
Arabinogalactan proteins - Multifunctional glycoproteins of the plant cell wall.Cell Surf. 2023 Feb 17;9:100102. doi: 10.1016/j.tcsw.2023.100102. eCollection 2023 Dec. Cell Surf. 2023. PMID: 36873729 Free PMC article. Review.
Cited by
-
In vivo structural modification of type II arabinogalactans with fungal endo-β-1, 6-galactanase in Arabidopsis.Front Plant Sci. 2022 Nov 9;13:1010492. doi: 10.3389/fpls.2022.1010492. eCollection 2022. Front Plant Sci. 2022. PMID: 36438144 Free PMC article.
-
Determining the Role of OsAGP6P in Anther Development Within the Arabinogalactan Peptide Family of Rice (Oryza sativa).Int J Mol Sci. 2025 Mar 14;26(6):2616. doi: 10.3390/ijms26062616. Int J Mol Sci. 2025. PMID: 40141257 Free PMC article.
-
Mechanisms of Plant Responses and Adaptation to Soil Salinity.Innovation (Camb). 2020 Apr 24;1(1):100017. doi: 10.1016/j.xinn.2020.100017. eCollection 2020 May 21. Innovation (Camb). 2020. PMID: 34557705 Free PMC article. Review.
-
Genome-wide identification and characterization of the FLA gene family in sorghum under salt-alkali stress.3 Biotech. 2025 May;15(5):117. doi: 10.1007/s13205-025-04283-9. Epub 2025 Apr 6. 3 Biotech. 2025. PMID: 40201754
-
New insights into bryophyte arabinogalactan-proteins from a hornwort and a moss model organism.Plant J. 2025 Jul;123(1):e70312. doi: 10.1111/tpj.70312. Plant J. 2025. PMID: 40589202 Free PMC article.
References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Stat. Methodol. 57, 289–300. Available online at: http://www.jstor.org/stable/2346101
-
- Darjania L., Ichise N., Ichikawa S., Okamoto T., Okuyama H., Thompson G. A. (2002). Dynamic turnover of arabinogalactan proteins in cultured Arabidopsis cells. Plant Physiol. Biochem. 40, 69–79. 10.1016/S0981-9428(01)01336-5 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

