Aqueous Photocurrent Measurements Correlated to Ultrafast Electron Transfer Dynamics at Ruthenium Tris Diimine-Sensitized NiO Photocathodes
- PMID: 28676835
- PMCID: PMC5493983
- DOI: 10.1021/acs.jpcc.6b12536
Aqueous Photocurrent Measurements Correlated to Ultrafast Electron Transfer Dynamics at Ruthenium Tris Diimine-Sensitized NiO Photocathodes
Abstract
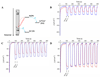
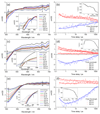
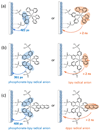
Understanding the structural and electronic factors governing the efficiency of dye-sensitized NiO photocathodes is essential to optimize solar fuel production in photoelectrochemical cells (PECs). For these purpose, three different ruthenium dyes, bearing either two or four methylphosphonate anchoring groups and either a bipyridine or a dipyridophenazine ancillary ligand, were synthesized and grafted onto NiO films. These photoelectrodes were fully characterized by XPS, ToF-SIMS, UV-vis absorption, time-resolved emission and femtosecond transient absorption spectroscopies. Increasing the number of anchoring groups from two to four proved beneficial for the grafting efficiency. No significant modification of the electronic properties compared to the parent photosensitizer was observed, in accordance with the non-conjugated nature of the grafted linker. The photoelectrochemical activity of the dye-sensitized NiO electrodes was assessed in fully aqueous medium in the presence of an irreversible electron acceptor and photocurrents reaching 190 μA.cm-2 were recorded. The transient absorption study revealed the presence of two charge recombination pathways for each of the sensitizers and evidenced a stabilized charge separated state in the dppz derivative, supporting its superior photoelectrochemical activity.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures



Similar articles
-
Localizing the initial excitation - A case study on NiO photocathodes using Ruthenium dipyridophenazine complexes as sensitizers.Spectrochim Acta A Mol Biomol Spectrosc. 2021 May 5;252:119507. doi: 10.1016/j.saa.2021.119507. Epub 2021 Jan 30. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33578124
-
Hydrogen Production at a NiO Photocathode Based on a Ruthenium Dye-Cobalt Diimine Dioxime Catalyst Assembly: Insights from Advanced Spectroscopy and Post-operando Characterization.ACS Appl Mater Interfaces. 2021 Oct 27;13(42):49802-49815. doi: 10.1021/acsami.1c12138. Epub 2021 Oct 12. ACS Appl Mater Interfaces. 2021. PMID: 34637266
-
Ultrafast and slow charge recombination dynamics of diketopyrrolopyrrole-NiO dye sensitized solar cells.Phys Chem Chem Phys. 2016 Jul 21;18(27):18515-27. doi: 10.1039/c6cp01762b. Epub 2016 Jun 24. Phys Chem Chem Phys. 2016. PMID: 27338174
-
Photoelectrochemical hydrogen evolution using CdTexS1-x quantum dots as sensitizers on NiO photocathodes.Dalton Trans. 2021 Jan 19;50(2):696-704. doi: 10.1039/d0dt03567j. Dalton Trans. 2021. PMID: 33346259
-
Direct evidence of catalyst reduction on dye and catalyst co-sensitized NiO photocathodes by mid-infrared transient absorption spectroscopy.Chem Sci. 2018 May 8;9(22):4983-4991. doi: 10.1039/c8sc00990b. eCollection 2018 Jun 14. Chem Sci. 2018. PMID: 29938026 Free PMC article.
Cited by
-
Efficient Light-Driven Hydrogen Evolution Using a Thiosemicarbazone-Nickel (II) Complex.Front Chem. 2019 Jun 27;7:405. doi: 10.3389/fchem.2019.00405. eCollection 2019. Front Chem. 2019. PMID: 31316966 Free PMC article.
-
Photophysical Study on the Effect of the External Potential on NiO-Based Photocathodes.ACS Appl Mater Interfaces. 2024 Jan 31;16(4):5217-5224. doi: 10.1021/acsami.3c09566. Epub 2024 Jan 18. ACS Appl Mater Interfaces. 2024. PMID: 38235571 Free PMC article.
-
Structure of Ni(OH)2 intermediates determines the efficiency of NiO-based photocathodes - a case study using novel mesoporous NiO nanostars.RSC Adv. 2019 Nov 29;9(67):39422-39433. doi: 10.1039/c9ra08785k. eCollection 2019 Nov 27. RSC Adv. 2019. PMID: 35540634 Free PMC article.
-
Photocathodes beyond NiO: charge transfer dynamics in a π-conjugated polymer functionalized with Ru photosensitizers.Sci Rep. 2021 Feb 2;11(1):2787. doi: 10.1038/s41598-021-82395-x. Sci Rep. 2021. PMID: 33531588 Free PMC article.
-
An artificial photosynthetic system for photoaccumulation of two electrons on a fused dipyridophenazine (dppz)-pyridoquinolinone ligand.Chem Sci. 2018 Apr 2;9(17):4152-4159. doi: 10.1039/c7sc04348a. eCollection 2018 May 7. Chem Sci. 2018. PMID: 29780545 Free PMC article.
References
-
- Brennaman MK, Dillon RJ, Alibabaei L, Gish MK, Dares CJ, Ashford DL, House RL, Meyer GJ, Papanikolas JM, Meyer TJ. Finding the Way to Solar Fuels with Dye-Sensitized Photoelectrosynthesis Cells. J Am Chem Soc. 2016;138:13085–13102. - PubMed
-
- Queyriaux N, Kaeffer N, Morozan A, Chavarot-Kerlidou M, Artero V. Molecular cathode and photocathode materials for hydrogen evolution in photoelectrochemical devices. J Photochem Photobiol C. 2015;25:90–105.
-
- Ashford DL, Gish MK, Vannucci AK, Brennaman MK, Templeton JL, Papanikolas JM, Meyer TJ. Molecular Chromophore–Catalyst Assemblies for Solar Fuel Applications. Chem Rev. 2015;115:13006–13049. - PubMed
-
- Yu Z, Li F, Sun L. Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components. Energy Environ Sci. 2015;8:760–775.
-
- Odobel F, Pellegrin Y. Recent Advances in the Sensitization of Wide-Band-Gap Nanostructured p-Type Semiconductors. Photovoltaic and Photocatalytic Applications. J Phys Chem Lett. 2013;4:2551–2564.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
