In-Cell Western Assays to Evaluate Hantaan Virus Replication as a Novel Approach to Screen Antiviral Molecules and Detect Neutralizing Antibody Titers
- PMID: 28676847
- PMCID: PMC5476785
- DOI: 10.3389/fcimb.2017.00269
In-Cell Western Assays to Evaluate Hantaan Virus Replication as a Novel Approach to Screen Antiviral Molecules and Detect Neutralizing Antibody Titers
Abstract
Hantaviruses encompass rodent-borne zoonotic pathogens that cause severe hemorrhagic fever disease with high mortality rates in humans. Detection of infectious virus titer lays a solid foundation for virology and immunology researches. Canonical methods to assess viral titers rely on visible cytopathic effects (CPE), but Hantaan virus (HTNV, the prototype hantavirus) maintains a relatively sluggish life cycle and does not produce CPE in cell culture. Here, an in-cell Western (ICW) assay was utilized to rapidly measure the expression of viral proteins in infected cells and to establish a novel approach to detect viral titers. Compared with classical approaches, the ICW assay is accurate and time- and cost-effective. Furthermore, the ICW assay provided a high-throughput platform to screen and identify antiviral molecules. Potential antiviral roles of several DExD/H box helicase family members were investigated using the ICW assay, and the results indicated that DDX21 and DDX60 reinforced IFN responses and exerted anti-hantaviral effects, whereas DDX50 probably promoted HTNV replication. Additionally, the ICW assay was also applied to assess NAb titers in patients and vaccine recipients. Patients with prompt production of NAbs tended to have favorable disease outcomes. Modest NAb titers were found in vaccinees, indicating that current vaccines still require improvements as they cannot prime host humoral immunity with high efficiency. Taken together, our results indicate that the use of the ICW assay to evaluate non-CPE Hantaan virus titer demonstrates a significant improvement over current infectivity approaches and a novel technique to screen antiviral molecules and detect NAb efficacies.
Keywords: DDX21; DDX60; disease severity; hantaan virus; in-cell western assays; interferon; neutralizing antibody.
Figures
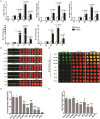

Similar articles
-
The Long Noncoding RNA NEAT1 Exerts Antihantaviral Effects by Acting as Positive Feedback for RIG-I Signaling.J Virol. 2017 Apr 13;91(9):e02250-16. doi: 10.1128/JVI.02250-16. Print 2017 May 1. J Virol. 2017. PMID: 28202761 Free PMC article.
-
Induction of Hantaan virus-specific immune responses in C57BL/6 mice by immunization with a modified recombinant adenovirus containing the chimeric gene, GcS0.7.Int J Mol Med. 2013 Sep;32(3):709-16. doi: 10.3892/ijmm.2013.1421. Epub 2013 Jun 20. Int J Mol Med. 2013. PMID: 23783439
-
DNA vaccination with the Hantaan virus M gene protects Hamsters against three of four HFRS hantaviruses and elicits a high-titer neutralizing antibody response in Rhesus monkeys.J Virol. 2001 Sep;75(18):8469-77. doi: 10.1128/jvi.75.18.8469-8477.2001. J Virol. 2001. PMID: 11507192 Free PMC article.
-
Hemorrhagic Fever with Renal Syndrome: Pathogenesis and Clinical Picture.Front Cell Infect Microbiol. 2016 Feb 3;6:1. doi: 10.3389/fcimb.2016.00001. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26870699 Free PMC article. Review.
-
Hantavirus: an overview and advancements in therapeutic approaches for infection.Front Microbiol. 2023 Oct 12;14:1233433. doi: 10.3389/fmicb.2023.1233433. eCollection 2023. Front Microbiol. 2023. PMID: 37901807 Free PMC article. Review.
Cited by
-
KIF26B and CREB3L1 Derived from Immunoscore Could Inhibit the Progression of Ovarian Cancer.J Immunol Res. 2024 Feb 13;2024:4817924. doi: 10.1155/2024/4817924. eCollection 2024. J Immunol Res. 2024. PMID: 38380081 Free PMC article.
-
Disparate macrophage responses are linked to infection outcome of Hantan virus in humans or rodents.Nat Commun. 2024 Jan 10;15(1):438. doi: 10.1038/s41467-024-44687-4. Nat Commun. 2024. PMID: 38200007 Free PMC article.
-
The DEAD-box RNA helicase, DDX60, Suppresses immunotherapy and promotes malignant progression of pancreatic cancer.Biochem Biophys Rep. 2023 May 26;34:101488. doi: 10.1016/j.bbrep.2023.101488. eCollection 2023 Jul. Biochem Biophys Rep. 2023. PMID: 37274827 Free PMC article.
-
An Improved Enzyme-Linked Focus Formation Assay Revealed Baloxavir Acid as a Potential Antiviral Therapeutic Against Hantavirus Infection.Front Pharmacol. 2019 Oct 16;10:1203. doi: 10.3389/fphar.2019.01203. eCollection 2019. Front Pharmacol. 2019. PMID: 31680975 Free PMC article.
-
Nlrc3 Knockout Mice Showed Renal Pathological Changes After HTNV Infection.Front Immunol. 2021 Jul 16;12:692509. doi: 10.3389/fimmu.2021.692509. eCollection 2021. Front Immunol. 2021. PMID: 34335602 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

