Neuroinflammation and Infection: Molecular Mechanisms Associated with Dysfunction of Neurovascular Unit
- PMID: 28676848
- PMCID: PMC5476750
- DOI: 10.3389/fcimb.2017.00276
Neuroinflammation and Infection: Molecular Mechanisms Associated with Dysfunction of Neurovascular Unit
Abstract
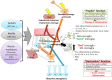
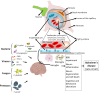
Neuroinflammation is a complex inflammatory process in the central nervous system, which is sought to play an important defensive role against various pathogens, toxins or factors that induce neurodegeneration. The onset of neurodegenerative diseases and various microbial infections are counted as stimuli that can challenge the host immune system and trigger the development of neuroinflammation. The homeostatic nature of neuroinflammation is essential to maintain the neuroplasticity. Neuroinflammation is regulated by the activity of neuronal, glial, and endothelial cells within the neurovascular unit, which serves as a "platform" for the coordinated action of pro- and anti-inflammatory mechanisms. Production of inflammatory mediators (cytokines, chemokines, reactive oxygen species) by brain resident cells or cells migrating from the peripheral blood, results in the impairment of blood-brain barrier integrity, thereby further affecting the course of local inflammation. In this review, we analyzed the most recent data on the central nervous system inflammation and focused on major mechanisms of neurovascular unit dysfunction caused by neuroinflammation and infections.
Keywords: blood-brain barrier; brain development; immune response; infectious diseases; neurodegeneration.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

