Exploring the Interplay between Rescue Drugs, Data Imputation, and Study Outcomes: Conceptual Review and Qualitative Analysis of an Acute Pain Data Set
- PMID: 28676997
- PMCID: PMC5693805
- DOI: 10.1007/s40122-017-0074-5
Exploring the Interplay between Rescue Drugs, Data Imputation, and Study Outcomes: Conceptual Review and Qualitative Analysis of an Acute Pain Data Set
Erratum in
-
Erratum to: Exploring the Interplay between Rescue Drugs, Data Imputation, and Study Outcomes: Conceptual Review and Qualitative Analysis of an Acute Pain Data Set.Pain Ther. 2017 Dec;6(2):177-178. doi: 10.1007/s40122-017-0078-1. Pain Ther. 2017. PMID: 28853028 Free PMC article. No abstract available.
Abstract
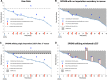
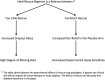
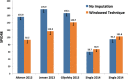
In placebo-controlled acute surgical pain studies, provisions must be made for study subjects to receive adequate analgesic therapy. As such, most protocols allow study subjects to receive a pre-specified regimen of open-label analgesic drugs (rescue drugs) as needed. The selection of an appropriate rescue regimen is a critical experimental design choice. We hypothesized that a rescue regimen that is too liberal could lead to all study arms receiving similar levels of pain relief (thereby confounding experimental results), while a regimen that is too stringent could lead to a high subject dropout rate (giving rise to a preponderance of missing data). Despite the importance of rescue regimen as a study design feature, there exist no published review articles or meta-analysis focusing on the impact of rescue therapy on experimental outcomes. Therefore, when selecting a rescue regimen, researchers must rely on clinical factors (what analgesics do patients usually receive in similar surgical scenarios) and/or anecdotal evidence. In the following article, we attempt to bridge this gap by reviewing and discussing the experimental impacts of rescue therapy on a common acute surgical pain population: first metatarsal bunionectomy. The function of this analysis is to (1) create a framework for discussion and future exploration of rescue as a methodological study design feature, (2) discuss the interplay between data imputation techniques and rescue drugs, and (3) inform the readership regarding the impact of data imputation techniques on the validity of study conclusions. Our findings indicate that liberal rescue may degrade assay sensitivity, while stringent rescue may lead to unacceptably high dropout rates.
Keywords: Acute pain; Analgesia; Bunionectomy; Clinical trial design; Clinical trials; Data imputation; Missing data; Pain; Post-surgical pain; Postoperative pain; Rescue medication.
Figures

References
-
- Apfelbaum J, Desjardins P, Brown M, Verburg K. Multiple-day efficacy of parecoxib sodium treatment in postoperative bunionectomy pain. Pain. 2008;24(9):784–792. - PubMed
-
- Daniels S, Casson E, Stegmann J, Oh C, Okamoto A, Rauschkolb C, Upmalis D. A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr Med Res Opin. 2009;25(6):1551–1561. doi: 10.1185/03007990902952825. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

