Persistent impairments 3 years after (neo)adjuvant chemotherapy for breast cancer: results from the MaTox project
- PMID: 28677012
- PMCID: PMC5602000
- DOI: 10.1007/s10549-017-4365-7
Persistent impairments 3 years after (neo)adjuvant chemotherapy for breast cancer: results from the MaTox project
Abstract
Purpose: Although treatment for early breast cancer improved prognosis greatly, it can have significant long-term consequences, which must be considered during treatment decision.
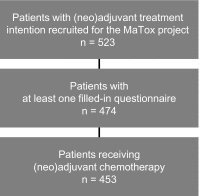
Methods: 453 patients with neoadjuvant or adjuvant treatment intention were recruited into the MaTox project within the prospective, multicentre, population-based German TMK cohort study (Tumour Registry Breast Cancer) between 2008 and 2009. Patient-reported outcomes (PROs) on 26 treatment-related symptoms were assessed via a specifically designed questionnaire at 4 weeks, 6 months, 18 months and 3 years after start of systemic treatment.
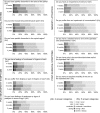
Results: The results show that alterations in smell, taste and appetite were clearly improved 3 years after treatment. In contrast, post-surgical symptoms, restrictions in memory/attention, musculoskeletal system and polyneuropathy worsened substantially over time and were persistent after 3 years: 78% of the patients recorded impairment in memory, 73% muscle pain, 67% pain at the operated site and 57% paraesthesia in fingers or toes. A logistic regression model showed that risk factors for developing persistent paraesthesia symptoms were age, early paraesthesia symptoms and taxane-based therapy.
Conclusions: Our data show that most patients with breast cancer have persistent impairments negatively influencing their daily life even 3 years after treatment. Furthermore, we highlight areas requiring special attention in follow-up care.
Keywords: Breast neoplasms; Chemotherapy, adjuvant; Cohort studies; Drug-related side effects and adverse reactions; Outpatients; Questionnaires.
Conflict of interest statement
Conflict of interest
H-JH, TG, UH, JH, LK and MJ declare no conflict of interest concerning the topic of this publication. NM and HT have received honoraria by Roche, Novartis, Celgene and Amgen for talks and attendance of conferences.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures

References
-
- Ferlay J, Soerjomataram I, Ervik M et al (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed 10 Aug 2016
-
- Robert Koch Institut . Krebs in Deutschland 2011/2012, 10. Ausgabe. Berlin: Robert Koch-Institut; 2015.
-
- Howlader N, Noone A, Krapcho M et al (2016) SEER Cancer Statistics Review, 1975-2013. Based on November 2015 SEER data submission, posted to the SEER web site, April 2016, http://seer.cancer.gov/csr/1975_2013/. National Cancer Institute, Bethesda, MD
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

