Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth
- PMID: 28677826
- PMCID: PMC6084289
- DOI: 10.1111/bjh.14787
Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth
Abstract
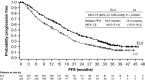
The randomized phase III ELOQUENT-2 study (NCT01239797) evaluated the efficacy and safety of elotuzumab + lenalidomide/dexamethasone (ELd) versus lenalidomide/dexamethasone (Ld) in relapsed/refractory multiple myeloma. ELd reduced the risk of disease progression/death by 30% versus Ld (hazard ratio [HR] 0·70). Median time from diagnosis was 3·5 years. We present extended 3-year follow-up data. Endpoints included progression-free survival (PFS), overall response rate (ORR) and interim overall survival (OS). Exploratory post-hoc analyses included impact of time from diagnosis and prior lines of therapy on PFS, and serum M-protein dynamic modelling. ORR was 79% (ELd) and 66% (Ld) (P = 0·0002). ELd reduced the risk of disease progression/death by 27% versus Ld (HR 0·73; P = 0·0014). Interim OS demonstrated a trend in favour of ELd (P = 0·0257); 1-, 2- and 3-year rates with ELd versus Ld were: 91% versus 83%, 73% versus 69% and 60% versus 53%. In patients with ≥ median time from diagnosis and one prior therapy, ELd resulted in a 53% reduction in the risk of progression/death versus Ld (HR 0·47). Serum M-protein dynamic modelling showed slower tumour regrowth with ELd. Adverse events were comparable between arms. ELd provided a durable and clinically relevant improvement in efficacy, with minimal incremental toxicity.
Keywords: elotuzumab; monoclonal antibody; multiple myeloma; overall survival; progression-free survival.
© 2017 John Wiley & Sons Ltd.
Figures






References
-
- Amgen (2016) FDA approves new Kyprolis® (carfilzomib) combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. Available at: http://www.amgen.com/media/news-releases/2016/01/fda-approves-new-kyprol.... (accessed 1 August 2016).
-
- Collins, S.M. , Bakan, C.E. , Swartzel, G.D. , Hofmeister, C.C. , Efebera, Y.A. , Kwon, H. , Starling, G.C. , Ciarlariello, D. , Bhaskar, S. , Briercheck, E.L. , Hughes, T. , Yu, J. , Rice, A. & Benson, D.M. Jr (2013) Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunology, Immunotherapy, 62, 1841–1849. - PMC - PubMed
-
- Dimopoulos, M.A. , Chen, C. , Spencer, A. , Niesvizky, R. , Attal, M. , Stadtmauer, E.A. , Petrucci, M.T. , Yu, Z. , Olesnyckyj, M. , Zeldis, J.B. , Knight, R.D. & Weber, D.M. (2009) Long‐term follow‐up on overall survival from the MM‐009 and MM‐010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia, 23, 2147–2152. - PubMed
-
- Dimopoulos, M.A. , Oriol, A. , Nahi, H. , San‐Miguel, J. , Bahlis, N.J. , Usmani, S.Z. , Rabin, N. , Orlowski, R.Z. , Komarnicki, M. , Suzuki, K. , Plesner, T. , Yoon, S.S. , Ben, Y.D. , Richardson, P.G. , Goldschmidt, H. , Reece, D. , Lisby, S. , Khokhar, N.Z. , O'Rourke, L. , Chiu, C. , Qin, X. , Guckert, M. , Ahmadi, T. & Moreau, P. (2016) Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. New England Journal of Medicine, 375, 1319–1331. - PubMed
-
- Durie, B.G. , Harousseau, J.L. , Miguel, J.S. , Blade, J. , Barlogie, B. , Anderson, K. , Gertz, M. , Dimopoulos, M. , Westin, J. , Sonneveld, P. , Ludwig, H. , Gahrton, G. , Beksac, M. , Crowley, J. , Belch, A. , Boccadaro, M. , Cavo, M. , Turesson, I. , Joshua, D. , Vesole, D. , Kyle, R. , Alexanian, R. , Tricot, G. , Attal, M. , Merlini, G. , Powles, R. , Richardson, P. , Shimizu, K. , Tosi, P. , Morgan, G. & Rajkumar, S.V. (2006) International uniform response criteria for multiple myeloma. Leukemia, 20, 1467–1473 [erratum in: Leukemia, 2007; 21, 1134; Leukemia, 2006; 20, 2220]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

