A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction
- PMID: 28677877
- PMCID: PMC6607490
- DOI: 10.1002/ejhf.897
A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction
Abstract
Aims: This study was designed to evaluate the safety, tolerability and haemodynamic effects of BMS-986231, a novel second-generation nitroxyl donor with potential inotropic, lusitropic and vasodilatory effects in patients hospitalized with decompensated heart failure and reduced ejection fraction (HFrEF).
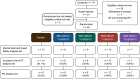
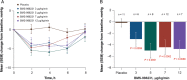
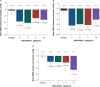
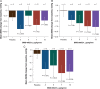
Methods and results: Forty-six patients hospitalized with decompensated HFrEF were enrolled into four sequential dose-escalation cohorts in this double-blind, randomized, placebo-controlled Phase 2a study. Patients with baseline pulmonary capillary wedge pressure (PCWP) of ≥20 mmHg and a cardiac index of ≤2.5 L/min/m2 received one 6-h i.v. infusion of BMS-986231 (at 3, 5, 7 or 12 µg/kg/min) or placebo. BMS-986231 produced rapid and sustained reductions in PCWP, as well as consistent reductions in time-averaged pulmonary arterial systolic pressure, pulmonary arterial diastolic pressure and right atrial pressure. BMS-986231 increased non-invasively measured time-averaged stroke volume index, cardiac index and cardiac power index values, and decreased total peripheral vascular resistance. There was no evidence of increased heart rate, drug-related arrhythmia or symptomatic hypotension with BMS-986231. Analyses of adverse events throughout the 30-day follow-up did not identify any toxicities specific to BMS-986231, with the potential exception of infrequent mild-to-moderate headaches during infusion. There were no treatment-related serious adverse events.
Conclusions: BMS-986231 demonstrated a favourable safety and haemodynamic profile in patients hospitalized with advanced heart failure. Based on preclinical data and these study's findings, it is possible that the haemodynamic benefits may be mediated by inotropic and/or lusitropic as well as vasodilatory effects. The therapeutic potential of BMS-986231 should be further assessed in patients with heart failure.
Keywords: BMS-986231; CXL-1427; Heart failure; Human; Nitroxyl.
© 2017 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures
Comment in
-
Nitroxyl donors for acute heart failure: promising newcomers.Eur J Heart Fail. 2017 Oct;19(10):1333-1334. doi: 10.1002/ejhf.793. Epub 2017 Aug 14. Eur J Heart Fail. 2017. PMID: 28805989 No abstract available.
References
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. - PubMed
-
- Greenberg B. Acute decompensated heart failure: treatments and challenges. Circ J 2012;76:532–543. - PubMed
-
- Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res 2007;100:96–104. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

