Characterization of Danaparoid Complex Extractive Drug by an Orthogonal Analytical Approach
- PMID: 28678201
- PMCID: PMC6152146
- DOI: 10.3390/molecules22071116
Characterization of Danaparoid Complex Extractive Drug by an Orthogonal Analytical Approach
Abstract
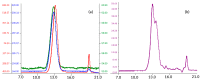
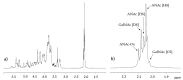
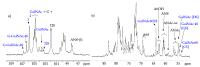
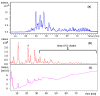
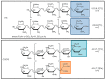
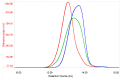
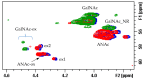
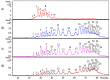
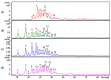
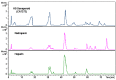
Danaparoid sodium salt, is the active component of ORGARAN, an anticoagulant and antithrombotic drug constituted of three glycosaminoglycans (GAGs) obtained from porcine intestinal mucosa extracts. Heparan sulfate is the major component, dermatan sulfate and chondroitin sulfate being the minor ones. Currently dermatan sulfate and chondroitin sulfate are quantified by UV detection of their unsaturated disaccharides obtained by enzymatic depolymerization. Due to the complexity of danaparoid biopolymers and the presence of shared components, an orthogonal approach has been applied using more advanced tools and methods. To integrate the analytical profile, 2D heteronuclear single quantum coherence (HSQC) NMR spectroscopy was applied and found effective to identify and quantify GAG component signals as well as those of some process signatures of danaparoid active pharmaceutical ingredient (API) batches. Analyses of components of both API samples and size separated fractions proceeded through the determination and distribution of the molecular weight (Mw) by high performance size exclusion chromatographic triple detector array (HP-SEC-TDA), chain mapping by LC/MS, and mono- (¹H and 13C) and bi-dimensional (HSQC) NMR spectroscopy. Finally, large scale chromatographic isolation and depolymerization of each GAG followed by LC/MS and 2D-NMR analysis, allowed the sequences to be defined and components to be evaluated of each GAG including oxidized residues of hexosamines and uronic acids at the reducing ends.
Keywords: component quantitative analysis; danaparoid sodium; low molecular weight glycosaminoglycans; orthogonal multi-analytical methods; sequence and compositional investigations.
Conflict of interest statement
R.v.H and P.d.W. declare that they are employed by Aspen Oss B.V. and that the study was paid for by Aspen Oss B.V.
Figures




References
-
- Council of Europe. European Pharmacopoeia Commission . European Pharmacopoeia. 6th ed. Strasbourg Council of Europe; Strasbourg, France: 2007. pp. 1644–1646.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

