The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells
- PMID: 28678424
- PMCID: PMC5756133
- DOI: 10.1002/cyto.a.23165
The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells
Abstract
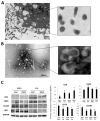
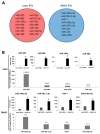
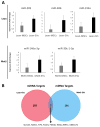
Autologous transplantation of mesenchymal stem cells (MSCs) is a viable option for the treatment of several diseases. Evidence indicates that MSCs release extracellular vesicles (EVs) and that EVs shuttle miRNAs to damaged parenchymal cells to activate an endogenous repair program. We hypothesize that comorbidities may interfere with the packaging of cargo in MSC-derived EVs. Therefore, we examined whether metabolic syndrome (MetS) modulates the miRNA content packed within MSC-derived EVs. MSCs were collected from swine abdominal adipose tissue after 16 weeks of lean or obese diet (n = 7 each). Next-generation RNA sequencing of miRNAs (miRNA-seq) was performed to identify miRNAs enriched in MSC-derived EVs and their predicted target genes. Functional pathway analysis of the top 50 target genes of the top 4 miRNAs enriched in each group was performed using gene ontology analysis. Lean- and MetS-EVs were enriched in, respectively, 14 and 8 distinct miRNAs. Target genes of miRNAs enriched in MetS-EVs were implicated in the development of MetS and its complications, including diabetes-related pathways, validated transcriptional targets of AP1 family members Fra1 and Fra2, Class A/1 (Rhodopsin-like receptors), and Peptide ligand-binding receptors. In contrast, miRNAs enriched in Lean EVs target primarily EphrinA-EPHA and the Rho family of GTPases. MetS alters the miRNA content of EVs derived from porcine adipose tissue MSCs. These alterations could impair the efficacy and limit the therapeutic use of autologous MSCs in subjects with MetS. Our findings may assist in developing adequate regenerative strategies to preserve the reparative potency of MSCs in individuals with MetS. © 2017 International Society for Advancement of Cytometry.
Keywords: extracellular vesicles; mesenchymal stem cells; metabolic syndrome; microRNA.
© 2017 International Society for Advancement of Cytometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Obesity reshapes stem cell extracellular vesicles.Cytometry A. 2018 Feb;93(2):177-179. doi: 10.1002/cyto.a.23166. Epub 2017 Jul 12. Cytometry A. 2018. PMID: 28700124 No abstract available.
Similar articles
-
Metabolic syndrome increases senescence-associated micro-RNAs in extracellular vesicles derived from swine and human mesenchymal stem/stromal cells.Cell Commun Signal. 2020 Aug 12;18(1):124. doi: 10.1186/s12964-020-00624-8. Cell Commun Signal. 2020. PMID: 32787856 Free PMC article.
-
Micro-RNAS Regulate Metabolic Syndrome-induced Senescence in Porcine Adipose Tissue-derived Mesenchymal Stem Cells through the P16/MAPK Pathway.Cell Transplant. 2018 Oct;27(10):1495-1503. doi: 10.1177/0963689718795692. Epub 2018 Sep 6. Cell Transplant. 2018. PMID: 30187775 Free PMC article.
-
Metabolic Syndrome Interferes with Packaging of Proteins within Porcine Mesenchymal Stem Cell-Derived Extracellular Vesicles.Stem Cells Transl Med. 2019 May;8(5):430-440. doi: 10.1002/sctm.18-0171. Epub 2019 Feb 1. Stem Cells Transl Med. 2019. PMID: 30707002 Free PMC article. Clinical Trial.
-
Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs.Stem Cell Res Ther. 2018 Nov 21;9(1):320. doi: 10.1186/s13287-018-1069-9. Stem Cell Res Ther. 2018. PMID: 30463593 Free PMC article. Review.
-
Mesenchymal Stromal Cell-Derived Extracellular Vesicles Regulate the Mitochondrial Metabolism via Transfer of miRNAs.Front Immunol. 2021 Mar 16;12:623973. doi: 10.3389/fimmu.2021.623973. eCollection 2021. Front Immunol. 2021. PMID: 33796099 Free PMC article. Review.
Cited by
-
Urinary Biomarkers for the Noninvasive Detection of Gastric Cancer.J Gastric Cancer. 2022 Oct;22(4):306-318. doi: 10.5230/jgc.2022.22.e28. J Gastric Cancer. 2022. PMID: 36316107 Free PMC article. Review.
-
Metabolic Syndrome Induces Release of Smaller Extracellular Vesicles from Porcine Mesenchymal Stem Cells.Cell Transplant. 2019 Sep-Oct;28(9-10):1271-1278. doi: 10.1177/0963689719860840. Epub 2019 Jun 28. Cell Transplant. 2019. PMID: 31250656 Free PMC article.
-
A Novel Nonsense INS Mutation Causes Inefficient Preproinsulin Translocation Into the Endoplasmic Reticulum.Front Endocrinol (Lausanne). 2022 Jan 5;12:774634. doi: 10.3389/fendo.2021.774634. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35069438 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges.Stem Cell Res Ther. 2017 Dec 4;8(1):273. doi: 10.1186/s13287-017-0727-7. Stem Cell Res Ther. 2017. PMID: 29202871 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Regulatory T Cells to Ameliorate Chronic Kidney Injury.Hypertension. 2020 May;75(5):1223-1232. doi: 10.1161/HYPERTENSIONAHA.119.14546. Epub 2020 Mar 30. Hypertension. 2020. PMID: 32223383 Free PMC article.
References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. - PubMed
-
- Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–92. - PubMed
-
- Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–41. - PubMed
-
- Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One. 2017;12(3):e0174303. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

