Transcription elongation factors represent in vivo cancer dependencies in glioblastoma
- PMID: 28678782
- PMCID: PMC5896562
- DOI: 10.1038/nature23000
Transcription elongation factors represent in vivo cancer dependencies in glioblastoma
Abstract
Glioblastoma is a universally lethal cancer with a median survival time of approximately 15 months. Despite substantial efforts to define druggable targets, there are no therapeutic options that notably extend the lifespan of patients with glioblastoma. While previous work has largely focused on in vitro cellular models, here we demonstrate a more physiologically relevant approach to target discovery in glioblastoma. We adapted pooled RNA interference (RNAi) screening technology for use in orthotopic patient-derived xenograft models, creating a high-throughput negative-selection screening platform in a functional in vivo tumour microenvironment. Using this approach, we performed parallel in vivo and in vitro screens and discovered that the chromatin and transcriptional regulators needed for cell survival in vivo are non-overlapping with those required in vitro. We identified transcription pause-release and elongation factors as one set of in vivo-specific cancer dependencies, and determined that these factors are necessary for enhancer-mediated transcriptional adaptations that enable cells to survive the tumour microenvironment. Our lead hit, JMJD6, mediates the upregulation of in vivo stress and stimulus response pathways through enhancer-mediated transcriptional pause-release, promoting cell survival specifically in vivo. Targeting JMJD6 or other identified elongation factors extends survival in orthotopic xenograft mouse models, suggesting that targeting transcription elongation machinery may be an effective therapeutic strategy for glioblastoma. More broadly, this study demonstrates the power of in vivo phenotypic screening to identify new classes of 'cancer dependencies' not identified by previous in vitro approaches, and could supply new opportunities for therapeutic intervention.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
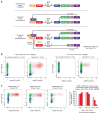



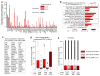









Comment in
-
Cancer: Keeping it real to kill glioblastoma.Nature. 2017 Jul 20;547(7663):291-292. doi: 10.1038/nature23095. Epub 2017 Jul 5. Nature. 2017. PMID: 28678780 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA169117/CA/NCI NIH HHS/United States
- TL1 TR002549/TR/NCATS NIH HHS/United States
- CA169117/NH/NIH HHS/United States
- NS087913/NH/NIH HHS/United States
- P30 CA043703/CA/NCI NIH HHS/United States
- CA189647/NH/NIH HHS/United States
- CA197718/NH/NIH HHS/United States
- R01 NS087913/NS/NINDS NIH HHS/United States
- R01 NS103434/NS/NINDS NIH HHS/United States
- F30 CA183510/CA/NCI NIH HHS/United States
- CA183510/NH/NIH HHS/United States
- NS089272/NH/NIH HHS/United States
- TL1 TR000441/TR/NCATS NIH HHS/United States
- R01 CA171652/CA/NCI NIH HHS/United States
- R01 NS089272/NS/NINDS NIH HHS/United States
- R35 CA197718/CA/NCI NIH HHS/United States
- F32 CA189647/CA/NCI NIH HHS/United States
- GM007250/NH/NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
- R01 CA154130/CA/NCI NIH HHS/United States
- CA171652/NH/NIH HHS/United States
- CA154130/NH/NIH HHS/United States
- F30 CA203101/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

