Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
- PMID: 28678794
- PMCID: PMC5497958
- DOI: 10.1371/journal.pmed.1002310
Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
Abstract
Background: Early revascularization of ischemic organs is key to improving outcomes, yet consequent reperfusion injury may be harmful. Reperfusion injury is largely attributed to excess mitochondrial production of reactive oxygen species (ROS). Sulfide inhibits mitochondria and reduces ROS production. Ammonium tetrathiomolybdate (ATTM), a copper chelator, releases sulfide in a controlled and novel manner, and may offer potential therapeutic utility.
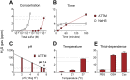
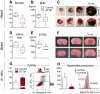
Methods and findings: In vitro, ATTM releases sulfide in a time-, pH-, temperature-, and thiol-dependent manner. Controlled sulfide release from ATTM reduces metabolism (measured as oxygen consumption) both in vivo in awake rats and ex vivo in skeletal muscle tissue, with a superior safety profile compared to standard sulfide generators. Given intravenously at reperfusion/resuscitation to rats, ATTM significantly reduced infarct size following either myocardial or cerebral ischemia, and conferred survival benefit following severe hemorrhage. Mechanistic studies (in vitro anoxia/reoxygenation) demonstrated a mitochondrial site of action (decreased MitoSOX fluorescence), where the majority of damaging ROS is produced.
Conclusions: The inorganic thiometallate ATTM represents a new class of sulfide-releasing drugs. Our findings provide impetus for further investigation of this compound as a novel adjunct therapy for reperfusion injury.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Magnus Oxygen Ltd are developing ATTM for the treatment of reperfusion injury. AD, JFM and MS are shareholders and MS is head of Magnus Oxygen. AD and ABL are former employees of Magnus Life Science, an historic service platform for Magnus Oxygen. MS is a member of the Editorial Board of PLoS Medicine.
Figures




References
-
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

