Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke
- PMID: 28678851
- PMCID: PMC5497997
- DOI: 10.1371/journal.pone.0180092
Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke
Abstract
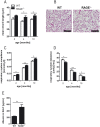
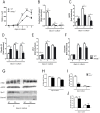
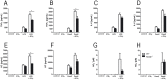
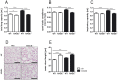
The receptor for advanced glycation endproducts (RAGE) is highly expressed in the lung but its physiological functions in this organ is still not completely understood. To determine the contribution of RAGE to physiological functions of the lung, we analyzed pulmonary mechanics and structure of wildtype and RAGE deficient (RAGE-/-) mice. RAGE deficiency spontaneously resulted in a loss of lung structure shown by an increased mean chord length, increased respiratory system compliance, decreased respiratory system elastance and increased concentrations of serum protein albumin in bronchoalveolar lavage fluids. Pulmonary expression of RAGE was mainly localized on alveolar epithelial cells and alveolar macrophages. Primary murine alveolar epithelial cells isolated from RAGE-/- mice revealed an altered differentiation and defective barrier formation under in vitro conditions. Stimulation of interferone-y (IFNy)-activated alveolar macrophages deficient for RAGE with Toll-like receptor (TLR) ligands resulted in significantly decreased release of proinflammatory cytokines and chemokines. Exposure to chronic cigarette smoke did not affect emphysema-like changes in lung parenchyma in RAGE-/- mice. Acute cigarette smoke exposure revealed a modified inflammatory response in RAGE-/- mice that was characterized by an influx of macrophages and a decreased keratinocyte-derived chemokine (KC) release. Our data suggest that RAGE regulates the differentiation of alveolar epithelial cells and impacts on the development and maintenance of pulmonary structure. In cigarette smoke-induced lung pathology, RAGE mediates inflammation that contributes to lung damage.
Conflict of interest statement
Figures

Similar articles
-
Emphysema requires the receptor for advanced glycation end-products triggering on structural cells.Am J Respir Cell Mol Biol. 2015 Apr;52(4):482-91. doi: 10.1165/rcmb.2014-0027OC. Am J Respir Cell Mol Biol. 2015. PMID: 25188021
-
Receptors for advanced glycation end-products targeting protect against hyperoxia-induced lung injury in mice.Am J Respir Cell Mol Biol. 2010 May;42(5):545-51. doi: 10.1165/rcmb.2008-0265OC. Epub 2009 Jun 18. Am J Respir Cell Mol Biol. 2010. PMID: 19541845
-
RAGE signaling by alveolar macrophages influences tobacco smoke-induced inflammation.Am J Physiol Lung Cell Mol Physiol. 2012 Jun 1;302(11):L1192-9. doi: 10.1152/ajplung.00099.2012. Epub 2012 Apr 13. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22505673
-
Plausible Roles for RAGE in Conditions Exacerbated by Direct and Indirect (Secondhand) Smoke Exposure.Int J Mol Sci. 2017 Mar 17;18(3):652. doi: 10.3390/ijms18030652. Int J Mol Sci. 2017. PMID: 28304347 Free PMC article. Review.
-
Increased surfactant protein-D and foamy macrophages in smoking-induced mouse emphysema.Respirology. 2007 Mar;12(2):191-201. doi: 10.1111/j.1440-1843.2006.01009.x. Respirology. 2007. PMID: 17298450 Review.
Cited by
-
Effects of PDE3 Inhibitor Olprinone on the Respiratory Parameters, Inflammation, and Apoptosis in an Experimental Model of Acute Respiratory Distress Syndrome.Int J Mol Sci. 2020 May 11;21(9):3382. doi: 10.3390/ijms21093382. Int J Mol Sci. 2020. PMID: 32403267 Free PMC article.
-
IL-17C contributes to NTHi-induced inflammation and lung damage in experimental COPD and is present in sputum during acute exacerbations.PLoS One. 2021 Jan 7;16(1):e0243484. doi: 10.1371/journal.pone.0243484. eCollection 2021. PLoS One. 2021. PMID: 33411748 Free PMC article.
-
HMGB1 participates in LPS‑induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF‑κB signaling pathways.Int J Mol Med. 2020 Jan;45(1):61-80. doi: 10.3892/ijmm.2019.4402. Epub 2019 Nov 12. Int J Mol Med. 2020. PMID: 31746367 Free PMC article.
-
The RAGE Axis: A Relevant Inflammatory Hub in Human Diseases.Biomolecules. 2024 Mar 28;14(4):412. doi: 10.3390/biom14040412. Biomolecules. 2024. PMID: 38672429 Free PMC article. Review.
-
Multiomics of World Trade Center Particulate Matter-induced Persistent Airway Hyperreactivity. Role of Receptor for Advanced Glycation End Products.Am J Respir Cell Mol Biol. 2020 Aug;63(2):219-233. doi: 10.1165/rcmb.2019-0064OC. Am J Respir Cell Mol Biol. 2020. PMID: 32315541 Free PMC article.
References
-
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(21):14998–5004. . - PubMed
-
- Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323(3):475–88. doi: 10.1007/s00441-005-0069-0 . - DOI - PubMed
-
- Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9(2):165–74. . - PubMed
-
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270(43):25752–61. . - PubMed
-
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

