The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication
- PMID: 28679761
- PMCID: PMC5571272
- DOI: 10.1128/JVI.00833-17
The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication
Abstract
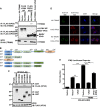
Ebola virus (EBOV), a member of the Filoviridae family, is a highly pathogenic virus that causes severe hemorrhagic fever in humans and is responsible for epidemics throughout sub-Saharan, central, and West Africa. The EBOV genome encodes VP35, an important viral protein involved in virus replication by acting as an essential cofactor of the viral polymerase as well as a potent antagonist of the host antiviral type I interferon (IFN-I) system. By using mass spectrometry analysis and coimmunoprecipitation assays, we show here that VP35 is ubiquitinated on lysine 309 (K309), a residue located on its IFN antagonist domain. We also found that VP35 interacts with TRIM6, a member of the E3-ubiquitin ligase tripartite motif (TRIM) family. We recently reported that TRIM6 promotes the synthesis of unanchored K48-linked polyubiquitin chains, which are not covalently attached to any protein, to induce efficient antiviral IFN-I-mediated responses. Consistent with this notion, VP35 also associated noncovalently with polyubiquitin chains and inhibited TRIM6-mediated IFN-I induction. Intriguingly, we also found that TRIM6 enhances EBOV polymerase activity in a minigenome assay and TRIM6 knockout cells have reduced replication of infectious EBOV, suggesting that VP35 hijacks TRIM6 to promote EBOV replication through ubiquitination. Our work provides evidence that TRIM6 is an important host cellular factor that promotes EBOV replication, and future studies will focus on whether TRIM6 could be targeted for therapeutic intervention against EBOV infection.IMPORTANCE EBOV belongs to a family of highly pathogenic viruses that cause severe hemorrhagic fever in humans and other mammals with high mortality rates (40 to 90%). Because of its high pathogenicity and lack of licensed antivirals and vaccines, EBOV is listed as a tier 1 select-agent risk group 4 pathogen. An important mechanism for the severity of EBOV infection is its suppression of innate immune responses. The EBOV VP35 protein contributes to pathogenesis, because it serves as an essential cofactor of the viral polymerase as well as a potent antagonist of innate immunity. However, how VP35 function is regulated by host cellular factors is poorly understood. Here, we report that the host E3-ubiquitin ligase TRIM6 promotes VP35 ubiquitination and is important for efficient virus replication. Therefore, our study identifies a new host factor, TRIM6, as a potential target in the development of antiviral drugs against EBOV.
Keywords: Ebola virus; TRIM6; VP35; innate immunity; tripartite motif (TRIM) protein; ubiquitination; unanchored ubiquitin; viral RNA polymerase; virus-host interactions.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, Palacios G, Peters CJ, Tenorio A, Volchkov VE, Jahrling PB. 2010. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol 155:2083–2103. doi: 10.1007/s00705-010-0814-x. - DOI - PMC - PubMed
-
- Sanchez AGT, Feldmann H. 2006. Filoviridae: Marburg and Ebola viruses, p 1409–1448. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

