Envelope contributions to the representation of interaural time difference in the forebrain of barn owls
- PMID: 28679844
- PMCID: PMC5599666
- DOI: 10.1152/jn.01166.2015
Envelope contributions to the representation of interaural time difference in the forebrain of barn owls
Abstract
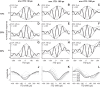
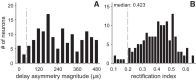
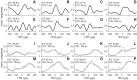
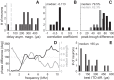
Birds and mammals use the interaural time difference (ITD) for azimuthal sound localization. While barn owls can use the ITD of the stimulus carrier frequency over nearly their entire hearing range, mammals have to utilize the ITD of the stimulus envelope to extend the upper frequency limit of ITD-based sound localization. ITD is computed and processed in a dedicated neural circuit that consists of two pathways. In the barn owl, ITD representation is more complex in the forebrain than in the midbrain pathway because of the combination of two inputs that represent different ITDs. We speculated that one of the two inputs includes an envelope contribution. To estimate the envelope contribution, we recorded ITD response functions for correlated and anticorrelated noise stimuli in the barn owl's auditory arcopallium. Our findings indicate that barn owls, like mammals, represent both carrier and envelope ITDs of overlapping frequency ranges, supporting the hypothesis that carrier and envelope ITD-based localization are complementary beyond a mere extension of the upper frequency limit.NEW & NOTEWORTHY The results presented in this study show for the first time that the barn owl is able to extract and represent the interaural time difference (ITD) information conveyed by the envelope of a broadband acoustic signal. Like mammals, the barn owl extracts the ITD of the envelope and the carrier of a signal from the same frequency range. These results are of general interest, since they reinforce a trend found in neural signal processing across different species.
Keywords: auditory arcopallium; carrier; envelope; extracellular recordings; sound localization.
Copyright © 2017 the American Physiological Society.
Figures









References
-
- Albeck Y, Konishi M. Responses of neurons in the auditory pathway of the barn owl to partially correlated binaural signals. J Neurophysiol 74: 1689–1700, 1995. - PubMed
-
- Batra R, Kuwada S, Stanford TR. High-frequency neurons in the inferior colliculus that are sensitive to interaural delays of amplitude-modulated tones: evidence for dual binaural influences. J Neurophysiol 70: 64–80, 1993. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

