Current treatment approaches for NK/T-cell lymphoma
- PMID: 28679966
- PMCID: PMC6144191
- DOI: 10.3960/jslrt.17018
Current treatment approaches for NK/T-cell lymphoma
Abstract
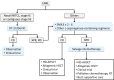
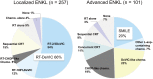
Extranodal NK/T-cell lymphoma, nasal type (ENKL), is a form of lymphoma characterized by preferential extranodal involvement, Epstein-Barr virus (EBV) association, and geographic diversity in incidence. ENKL tumor cells express P-glycoprotein, which is related to multidrug resistance (MDR). This MDR phenomenon is thought to be the major reason why ENKL is resistant to anthracycline-containing chemotherapies and has led researchers to explore novel therapeutic strategies. Since the early 2000s, next-generation therapies, including upfront radiotherapy, chemotherapy, or concurrent chemoradiotherapy using non-MDR-related drugs, have markedly changed the management of ENKL. However, a recent large retrospective study in Japan revealed several limitations of next-generation therapies, in particular that they resulted in almost no improvement of early disease progression. This review will summarize the current management of ENKL, primarily based on clinical trial results, and provide clues for better future management.
Keywords: NK/T-cell lymphoma; guidelines; prognostic model; treatment.
Conflict of interest statement
Figures

Similar articles
-
Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type.Blood. 2018 Jun 7;131(23):2528-2540. doi: 10.1182/blood-2017-12-791418. Epub 2018 Mar 30. Blood. 2018. PMID: 29602763 Review.
-
Epstein-Barr virus-associated natural killer/T-cell lymphomas.Best Pract Res Clin Haematol. 2013 Mar;26(1):15-21. doi: 10.1016/j.beha.2013.04.002. Epub 2013 May 25. Best Pract Res Clin Haematol. 2013. PMID: 23768637 Review.
-
Extranodal NK/T-cell lymphoma: Updates in biology and management strategies.Best Pract Res Clin Haematol. 2018 Sep;31(3):315-321. doi: 10.1016/j.beha.2018.07.002. Epub 2018 Jul 3. Best Pract Res Clin Haematol. 2018. PMID: 30213402 Review.
-
Current and future management of NK/T-cell lymphoma based on clinical trials.Int J Hematol. 2012 Nov;96(5):562-71. doi: 10.1007/s12185-012-1189-4. Epub 2012 Oct 17. Int J Hematol. 2012. PMID: 23073619 Review.
-
[Treatment of NK/T-cell lymphoma: current situations and prospectives].Rinsho Ketsueki. 2018;59(5):588-593. doi: 10.11406/rinketsu.59.588. Rinsho Ketsueki. 2018. PMID: 29877250 Japanese.
Cited by
-
EBV-Related Lymphoproliferative Diseases: A Review in Light of New Classifications.Mediterr J Hematol Infect Dis. 2024 May 1;16(1):e2024042. doi: 10.4084/MJHID.2024.042. eCollection 2024. Mediterr J Hematol Infect Dis. 2024. PMID: 38882456 Free PMC article. Review.
-
A case report of an extranodal NK/T-cell lymphoma nasal type, occurring primarily in eyes with masquerade syndrome.Medicine (Baltimore). 2019 Mar;98(11):e14836. doi: 10.1097/MD.0000000000014836. Medicine (Baltimore). 2019. PMID: 30882672 Free PMC article.
-
Novel findings from the Asian Lymphoma Study Group: focus on T and NK-cell lymphomas.Int J Hematol. 2018 Apr;107(4):413-419. doi: 10.1007/s12185-018-2406-6. Epub 2018 Jan 29. Int J Hematol. 2018. PMID: 29380182 Review.
-
Molecular Genetics in Epstein-Barr Virus-Associated Malignancies.Life (Basel). 2021 Jun 22;11(7):593. doi: 10.3390/life11070593. Life (Basel). 2021. PMID: 34206255 Free PMC article. Review.
-
Nasopharyngeal Lymphoma: A 22-Year Review of 35 Cases.J Clin Med. 2019 Oct 3;8(10):1604. doi: 10.3390/jcm8101604. J Clin Med. 2019. PMID: 31623372 Free PMC article.
References
-
- Swerdlow SH, Campo E, Harris NL, et al.: WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, IARC, 2008
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

