Synergistic cellular effects including mitochondrial destabilization, autophagy and apoptosis following low-level exposure to a mixture of lipophilic persistent organic pollutants
- PMID: 28680151
- PMCID: PMC5498599
- DOI: 10.1038/s41598-017-04654-0
Synergistic cellular effects including mitochondrial destabilization, autophagy and apoptosis following low-level exposure to a mixture of lipophilic persistent organic pollutants
Abstract
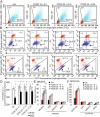
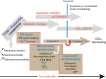
Humans are exposed to multiple exogenous environmental pollutants. Many of these compounds are parts of mixtures that can exacerbate harmful effects of the individual mixture components. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), is primarily produced via industrial processes including incineration and the manufacture of herbicides. Both endosulfan and TCDD are persistent organic pollutants which elicit cytotoxic effects by inducing reactive oxygen species generation. Sublethal concentrations of mixtures of TCDD and endosulfan increase oxidative stress, as well as mitochondrial homeostasis disruption, which is preceded by a calcium rise and, in fine, induce cell death. TCDD+Endosulfan elicit a complex signaling sequence involving reticulum endoplasmic destalilization which leads to Ca2+ rise, superoxide anion production, ATP drop and late NADP(H) depletion associated with a mitochondrial induced apoptosis concomitant early autophagic processes. The ROS scavenger, N-acetyl-cysteine, blocks both the mixture-induced autophagy and death. Calcium chelators act similarly and mitochondrially targeted anti-oxidants also abrogate these effects. Inhibition of the autophagic fluxes with 3-methyladenine, increases mixture-induced cell death. These findings show that subchronic doses of pollutants may act synergistically. They also reveal that the onset of autophagy might serve as a protective mechanism against ROS-triggered cytotoxic effects of a cocktail of pollutants in Caco-2 cells and increase their tumorigenicity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
-
- Parzefall, W. Risk assessment of dioxin contamination in human food. Food Chem Toxicol40, 1185–1189, doi:S0278691502000595 (2002). - PubMed
-
- Weber, J. et al. Endosulfan, a global pesticide: a review of its fate in the environment and occurrence in the Arctic. Sci Total Environ408, 2966–2984, doi:S0048-9697(09)01088-2 [pii] 10.1016/j.scitotenv.2009.10.077 (2010). - PubMed
-
- Barouki, R., Coumoul, X. & Fernandez-Salguero, P. M. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett581, 3608–3615, doi: S0014-5793(07)00317-1 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

