Syk Plays a Critical Role in the Expression and Activation of IRAK1 in LPS-Treated Macrophages
- PMID: 28680194
- PMCID: PMC5478860
- DOI: 10.1155/2017/1506248
Syk Plays a Critical Role in the Expression and Activation of IRAK1 in LPS-Treated Macrophages
Abstract
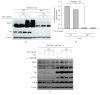
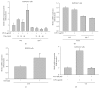
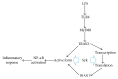
To address how interleukin-1 receptor-associated kinase 1 (IRAK1) is controlled by other enzymes activated by toll-like receptor (TLR) 4, we investigated the possibility that spleen tyrosine kinase (Syk), a protein tyrosine kinase that is activated at an earlier stage during TLR4 activation, plays a central role in regulating the functional activation of IRAK1. Indeed, we found that overexpression of myeloid differentiation primary response gene 88 (MyD88), an adaptor molecule that drives TLR signaling, induced IRAK1 expression and that piceatannol, a Syk inhibitor, successfully suppressed the MyD88-dependent upregulation of IRAK1 under LPS treatment conditions. Interestingly, in Syk-knockout RAW264.7 cells, IRAK1 activity was almost completely blocked after LPS treatment, while providing a Syk-recovery gene to the knockout cells successfully restored IRAK1 expression. According to our measurements of IRAK1 mRNA levels, the transcriptional upregulation of IRAK1 was induced by LPS treatment between 4 and 60 min, and this can be suppressed in Syk knockout cells, providing an effect similar that that seen under piceatannol treatment. The overexpression of Syk reverses this effect and leads to a significantly higher IRAK1 mRNA level. Collectively, our results strongly suggest that Syk plays a critical role in regulating both the activity and transcriptional level of IRAK1.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

