MYCN is an immunosuppressive oncogene dampening the expression of ligands for NK-cell-activating receptors in human high-risk neuroblastoma
- PMID: 28680748
- PMCID: PMC5486189
- DOI: 10.1080/2162402X.2017.1316439
MYCN is an immunosuppressive oncogene dampening the expression of ligands for NK-cell-activating receptors in human high-risk neuroblastoma
Abstract
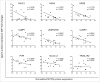
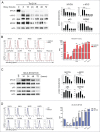
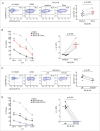
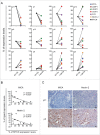
Neuroblastoma (NB) is the most common extracranial solid tumor occurring in childhood. Amplification of the MYCN oncogene is associated with poor prognosis. Downregulation on NB cells of ligands recognized by Natural Killer (NK) cell-activating receptors, involved in tumor cell recognition and lysis, may contribute to tumor progression and relapse. Here, we demonstrate that in human NB cell lines MYCN expression inversely correlates with that of ligands recognized by NKG2D and DNAM1 activating receptors in human NB cell lines. In the MYCN-inducible Tet-21/N cell line, downregulation of MYCN resulted in enhanced expression of the activating ligands MICA, ULBPs and PVR, which rendered tumor cells more susceptible to recognition and lysis mediated by NK cells. Conversely, a MYCN non-amplified NB cell line transfected with MYCN showed an opposite behavior compared with control cells. Consistent with these findings, an inverse correlation was detected between the expression of MYCN and that of ligands for NK-cell-activating receptors in 12 NB patient specimens both at mRNA and protein levels. Taken together, these results provide the first demonstration that MYCN acts as an immunosuppressive oncogene in NB cells that negatively regulates the expression of ligands for NKG2D and DNAM-1 NK-cell-activating receptors. Our study provides a clue to exploit MYCN expression levels as a biomarker to predict the efficacy of NK-cell-based immunotherapy in NB patients.
Keywords: Immunosuppressive oncogene; MYCN oncogene; NK-cell-activating receptor ligands; neuroblastoma; tumor immune escape.
Figures
References
-
- Maris JM. Recent advances in neuroblastoma. N Eng J Med 2010; 362:2202-11; PMID:20558371; https://doi.org/ 10.1056/NEJMra0804577 - DOI - PMC - PubMed
-
- Zimmerman KA, Yancopoulos GD, Collum RG, Smith RK, Kohl NE, Denis KA, Nau MM, Witte ON, Toran-Allerand D, Gee CE et al. Differential expression of MYC family genes during murine development. Nature 1986; 319:780-3; PMID:2419762; https://doi.org/ 10.1038/319780a0 - DOI - PubMed
-
- Raffaghello L, Prigione I, Bocca P, Morandi F, Camoriano M, Gambini C, Wang X, Ferrone S, Pistoia V. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene 2005; 24:4634-44; PMID:15897905; https://doi.org/ 10.1038/sj.onc.1208594 - DOI - PubMed
-
- Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, Wang L, Shifrin N, Raulet DH. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 2014; 122:91-128; PMID:24507156; https://doi.org/ 10.1016/B978-0-12-800267-4.00003-1 - DOI - PMC - PubMed
-
- Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S, Pistoia V. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 2004; 6:558-68; PMID:15548365; https://doi.org/ 10.1593/neo.04316 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
