Efficacy and safety of adding mizoribine to standard treatment in patients with immunoglobulin A nephropathy: A randomized controlled trial
- PMID: 28680823
- PMCID: PMC5491162
- DOI: 10.23876/j.krcp.2017.36.2.159
Efficacy and safety of adding mizoribine to standard treatment in patients with immunoglobulin A nephropathy: A randomized controlled trial
Abstract
Background: Mizoribine (MZR) is an immunosuppressive drug used in Japan for treating patients with lupus nephritis and nephrotic syndrome and has been also reportedly effective in patients with immunoglobulin A (IgA) nephropathy. However, to date, few randomized control studies of MZR are performed in patients with IgA nephropathy. Therefore, this prospective, open-label, randomized, controlled trial aimed to investigate the efficacy and safety of adding MZR to standard treatment in these patients, and was conducted between April 1, 2009, and March 31, 2016, as a multicenter study.
Methods: Patients were randomly assigned (1:1) to receiving standard treatment plus MZR (MZR group) or standard treatment (control group). MZR was administered orally at a dose of 150 mg once daily for 12 months.
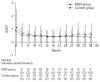
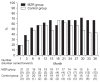
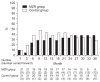
Results: Primary outcomes were the percentage reduction in urinary protein excretion from baseline and the rate of patients with hematuria disappearance 36 months after study initiation. Secondary outcomes were the rate of patients with proteinuria disappearance, clinical remission rate, absolute changes in estimated glomerular filtration rate from baseline, and the change in daily dose of prednisolone. Forty-two patients were randomly assigned to MZR (n = 21) and control groups (n = 21). Nine patients in MZR group and 15 patients in the control group completed the study. No significant differences were observed between the two groups with respect to primary and secondary outcomes.
Conclusion: The addition of MZR to standard treatment has no beneficial effect on reducing urinary protein excretion and hematuria when treating patients with IgA nephropathy.
Keywords: Hematuria; Immunoglobulin A nephropathy; Mizoribine.
Conflict of interest statement
Conflicts of interest All authors have no conflicts of interest to declare.
Figures



References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274.
-
- Li PK, Leung CB, Chow KM, Cheng YL, Fung SK, Mak SK, Tang AW, Wong TY, Yung CY, Yung JC, Yu AW, Szeto CC HKVIN Study Group. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47:751–760. doi: 10.1053/j.ajkd.2006.01.017. - DOI - PubMed
-
- Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Sekizuka K, Ebihara I, Koide H. Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgA nephropathy. Am J Nephrol. 2000;20:373–379. doi: 10.1159/000013619. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

