Behavior of Neutrophil Granulocytes during Toxoplasma gondii Infection in the Central Nervous System
- PMID: 28680853
- PMCID: PMC5478696
- DOI: 10.3389/fcimb.2017.00259
Behavior of Neutrophil Granulocytes during Toxoplasma gondii Infection in the Central Nervous System
Abstract
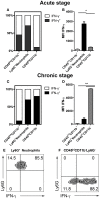
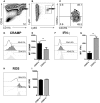
Cerebral toxoplasmosis is characterized by activation of brain resident cells and recruitment of specific immune cell subsets from the periphery to the central nervous system (CNS). Our studies revealed that the rapidly invaded Ly6G+ neutrophil granulocytes are an early non-lymphoid source of interferon-gamma (IFN-γ), the cytokine known to be the major mediator of host resistance to Toxoplasma gondii (T. gondii). Upon selective depletion of Ly6G+ neutrophils, we detected reduced IFN-γ production and increased parasite burden in the CNS. Ablation of Ly6G+ cells resulted in diminished recruitment of Ly6Chi monocytes into the CNS, indicating a pronounced interplay. Additionally, we identified infiltrated Ly6G+ neutrophils to be a heterogeneous population. The Ly6G+CD62-LhiCXCR4+ subset released cathelicidin-related antimicrobial peptide (CRAMP), which can promote monocyte dynamics. On the other hand, the Ly6G+CD62-LloCXCR4+ subset produced IFN-γ to establish early inflammatory response. Collectively, our findings revealed that the recruited Ly6G+CXCR4+ neutrophil granulocytes display a heterogeneity in the CNS with a repertoire of effector functions crucial in parasite control and immune regulation upon experimental cerebral toxoplasmosis.
Keywords: Toxoplasma gondii; cerebral toxoplasmosis; neuroinflammation; neutrophil Infiltration; neutrophil granulocytes.
Figures





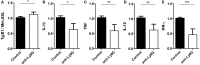
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

