A Population Pharmacokinetic Model to Predict the Individual Starting Dose of Tacrolimus Following Pediatric Renal Transplantation
- PMID: 28681225
- PMCID: PMC5856873
- DOI: 10.1007/s40262-017-0567-8
A Population Pharmacokinetic Model to Predict the Individual Starting Dose of Tacrolimus Following Pediatric Renal Transplantation
Abstract
Background: Multiple clinical, demographic, and genetic factors affect the pharmacokinetics of tacrolimus in children, yet in daily practice, a uniform body-weight based starting dose is used. It can take weeks to reach the target tacrolimus pre-dose concentration.
Objectives: The objectives of this study were to determine the pharmacokinetics of tacrolimus immediately after kidney transplantation and to find relevant parameters for dose individualization using a population pharmacokinetic analysis.
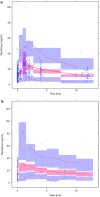
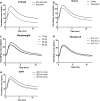
Methods: A total of 722 blood samples were collected from 46 children treated with tacrolimus over the first 6 weeks after renal transplantation. Non-linear mixed-effects modeling (NONMEM®) was used to develop a population pharmacokinetic model and perform a covariate analysis. Simulations were performed to determine the optimal starting dose and to develop dosing guidelines.
Results: The data were accurately described by a two-compartment model with allometric scaling for bodyweight. Mean tacrolimus apparent clearance was 50.5 L/h, with an inter-patient variability of 25%. Higher bodyweight, lower estimated glomerular filtration rate, and higher hematocrit levels resulted in lower total tacrolimus clearance. Cytochrome P450 3A5 expressers and recipients who received a kidney from a deceased donor had a significantly higher tacrolimus clearance. The model was successfully externally validated. In total, these covariates explained 41% of the variability in clearance. From the significant covariates, the cytochrome P450 3A5 genotype, bodyweight, and donor type were useful to adjust the starting dose to reach the target pre-dose concentration. Dosing guidelines range from 0.27 to 1.33 mg/kg/day.
Conclusion: During the first 6 weeks after transplantation, the tacrolimus weight-normalized starting dose should be higher in pediatric kidney transplant recipients with a lower bodyweight, those who express the cytochrome P450 3A5 genotype, and those who receive a kidney from a deceased donor.
Conflict of interest statement
Funding
No sources of funding were used in the preparation of this article.
Conflict of interest
Teun van Gelder has received a study Grant from Chiesi Pharmaceuticals, lecture fees from Chiesi Pharmaceuticals, Astellas Pharma, Roche Pharma, and Novartis Pharma, and consulting fees from Astellas Pharma, Novartis Pharma, and Teva Pharma. Dennis A. Hesselink has received grant support and lecture and consulting fees from Astellas Pharma and Chiesi Pharmaceuticals, as well as a lecture fee from Hikma Pharma. Brenda C. M. de Winter has received travel support from Astellas Pharma. Saskia N. de Wildt has received funding from The Netherlands Organisation for Health Research and Development (Grant No. 90700304). Louise M. Andrews, Roger J. M. Brüggemann, Elisabeth A. M. Cornelissen, Karlien Cransberg, Birgit C. P. Koch, and Ron H. N. van Schaik have no conflicts of interest directly relevant to the content of this article.
Figures

References
-
- Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

