Protein phosphatases 1 and 2A and their naturally occurring inhibitors: current topics in smooth muscle physiology and chemical biology
- PMID: 28681362
- PMCID: PMC5754374
- DOI: 10.1007/s12576-017-0556-6
Protein phosphatases 1 and 2A and their naturally occurring inhibitors: current topics in smooth muscle physiology and chemical biology
Erratum in
-
Correction to: Protein phosphatases 1 and 2A and their naturally occurring inhibitors: current topics in smooth muscle physiology and chemical biology.J Physiol Sci. 2018 Mar;68(2):201. doi: 10.1007/s12576-017-0577-1. J Physiol Sci. 2018. PMID: 29181704 Free PMC article.
Abstract
Protein phosphatases 1 and 2A (PP1 and PP2A) are the most ubiquitous and abundant serine/threonine phosphatases in eukaryotic cells. They play fundamental roles in the regulation of various cellular functions. This review focuses on recent advances in the functional studies of these enzymes in the field of smooth muscle physiology. Many naturally occurring protein phosphatase inhibitors with different relative PP1/PP2A affinities have been discovered and are widely used as powerful research tools. Current topics in the chemical biology of PP1/PP2A inhibitors are introduced and discussed, highlighting the identification of the gene cluster responsible for the biosynthesis of calyculin A in a symbiont microorganism of a marine sponge.
Keywords: Phosphatase inhibitors; Protein phosphatase 1; Protein phosphatase 2A; Protein phosphorylation; Signal transduction; Smooth muscle.
Figures
Similar articles
-
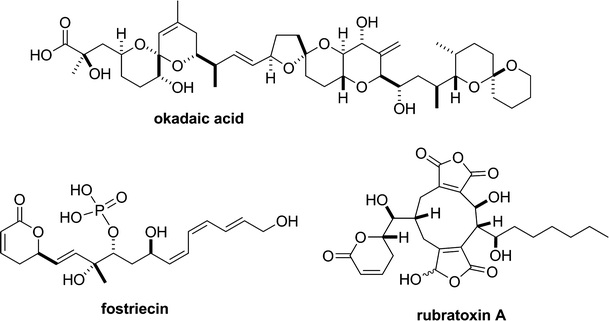
Structure-activity relationship studies of fostriecin, cytostatin, and key analogs, with PP1, PP2A, PP5, and( beta12-beta13)-chimeras (PP1/PP2A and PP5/PP2A), provide further insight into the inhibitory actions of fostriecin family inhibitors.J Pharmacol Exp Ther. 2009 Oct;331(1):45-53. doi: 10.1124/jpet.109.155630. Epub 2009 Jul 10. J Pharmacol Exp Ther. 2009. PMID: 19592665 Free PMC article.
-
Protein phosphatase 1, protein phosphatase 2A, and calcineurin play a role in estrogen-mediated neuroprotection.Endocrinology. 2008 Oct;149(10):5235-43. doi: 10.1210/en.2008-0610. Epub 2008 Jun 19. Endocrinology. 2008. PMID: 18566123 Free PMC article.
-
PP1/PP2A phosphatase inhibition-induced metaplasticity in protein synthesis blocker-treated hippocampal slices: LTP and LTD, or There and Back again.Biochem Biophys Res Commun. 2021 Jun 18;558:64-70. doi: 10.1016/j.bbrc.2021.04.061. Epub 2021 Apr 23. Biochem Biophys Res Commun. 2021. PMID: 33901925
-
Calyculins and related marine natural products as serine-threonine protein phosphatase PP1 and PP2A inhibitors and total syntheses of calyculin A, B, and C.Mar Drugs. 2010 Jan 21;8(1):122-72. doi: 10.3390/md80100122. Mar Drugs. 2010. PMID: 20161975 Free PMC article. Review.
-
cAMP regulation of protein phosphatases PP1 and PP2A in brain.Biochim Biophys Acta Mol Cell Res. 2019 Jan;1866(1):64-73. doi: 10.1016/j.bbamcr.2018.09.006. Epub 2018 Sep 18. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30401536 Free PMC article. Review.
Cited by
-
MX2-mediated innate immunity against HIV-1 is regulated by serine phosphorylation.Nat Microbiol. 2021 Aug;6(8):1031-1042. doi: 10.1038/s41564-021-00937-5. Epub 2021 Jul 19. Nat Microbiol. 2021. PMID: 34282309 Free PMC article.
-
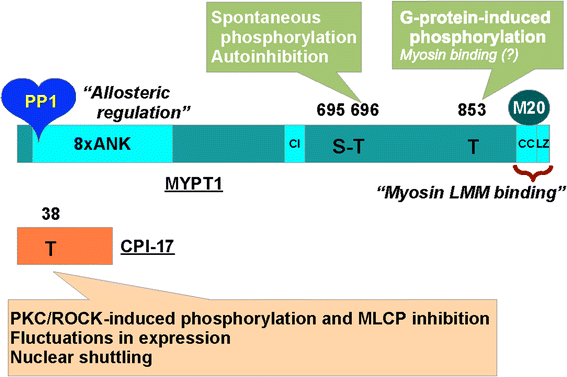
Possible roles of N- and C-terminal unstructured tails of CPI-17 in regulating Ca2+ sensitization force of smooth muscle.J Smooth Muscle Res. 2022;58(0):22-33. doi: 10.1540/jsmr.58.22. J Smooth Muscle Res. 2022. PMID: 35418530 Free PMC article.
-
The Cell Cycle Checkpoint System MAST(L)-ENSA/ARPP19-PP2A is Targeted by cAMP/PKA and cGMP/PKG in Anucleate Human Platelets.Cells. 2020 Feb 18;9(2):472. doi: 10.3390/cells9020472. Cells. 2020. PMID: 32085646 Free PMC article.
-
Cell-Penetrating Peptide Based on Myosin Phosphatase Target Subunit Sequence Mediates Myosin Phosphatase Activity.Biomolecules. 2025 May 12;15(5):705. doi: 10.3390/biom15050705. Biomolecules. 2025. PMID: 40427598 Free PMC article.
-
Inhibitory effects of rubratoxin A, a potent inhibitor of protein phosphatase 2, on the Ca2+-dependent contraction of skinned carotid artery from guinea pig.J Smooth Muscle Res. 2019;55(0):14-22. doi: 10.1540/jsmr.55.14. J Smooth Muscle Res. 2019. PMID: 31006724 Free PMC article.
References
-
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. - PubMed
-
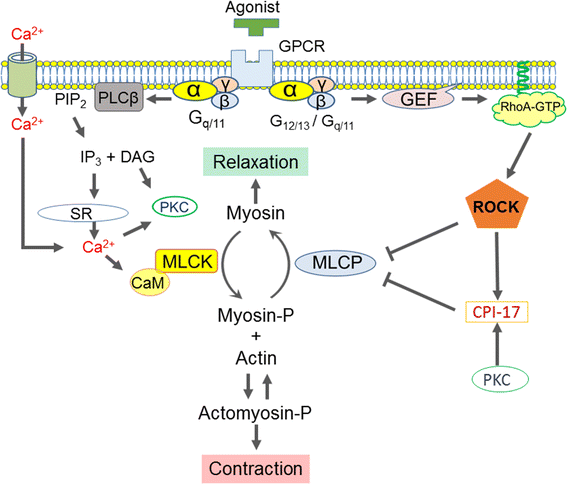
- Eto M, Kitazawa T, Murayama T. Manipulating temporal regulation of myosin phosphatase signaling. J Physiol Sci. 2016;66:S71.
-
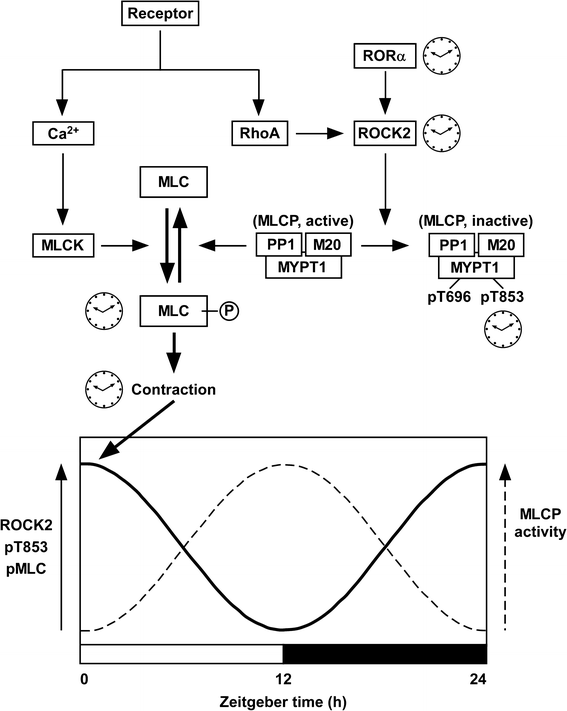
- Hirano K. Vascular intrinsic circadian rhythm of myosin phosphatase activity. J Physiol Sci. 2016;66:S71.
-
- Takeya K, Ishida M, Miyazu M, Takai A. Effects of PP2A inhibitors on smooth muscle contraction. J Physiol Sci. 2016;66:S71.
-
- Wakimoto T. Recent topics in natural product-derived protein phosphatase inhibitors. J Physiol Sci. 2016;66:S71.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

