Human monocytes downregulate innate response receptors following exposure to the microbial metabolite n-butyrate
- PMID: 28681454
- PMCID: PMC5691313
- DOI: 10.1002/iid3.184
Human monocytes downregulate innate response receptors following exposure to the microbial metabolite n-butyrate
Abstract
Introduction: Hyporesponsiveness of human lamina propria immune cells to microbial and nutritional antigens represents one important feature of intestinal homeostasis. It is at least partially mediated by low expression of the innate response receptors CD11b, CD14, CD16 as well as the cystine-glutamate transporter xCT on these cells. Milieu-specific mechanisms leading to the down-regulation of these receptors on circulating monocytes, the precursor cells of resident macrophages, are mostly unknown.
Methods: Here, we addressed the question whether the short chain fatty acid n-butyrate, a fermentation product of the mammalian gut microbiota exhibiting histone deacetylase inhibitory activity, is able to modulate expression of these receptors in human circulating monocytes.
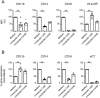
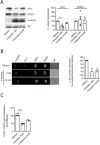
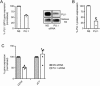
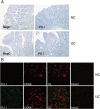
Results: Exposure to n-butyrate resulted in the downregulation of CD11b, CD14, as well as CD16 surface expression on circulating monocytes. XCT transcript levels in circulating monocytes were also reduced following exposure to n-butyrate. Importantly, treatment resulted in the downregulation of protein and gene expression of the transcription factor PU.1, which was shown to be at least partially required for the expression of CD16 in circulating monocytes. PU.1 expression in resident macrophages in situ was observed to be substantially lower in healthy when compared to inflamed colonic mucosa.
Conclusions: In summary, the intestinal microbiota may support symbiosis with the human host organism by n-butyrate mediated downregulation of protein and gene expression of innate response receptors as well as xCT on circulating monocytes following recruitment to the lamina propria. Downregulation of CD16 gene expression may at least partially be caused at the transcriptional level by the n-butyrate mediated decrease in expression of the transcription factor PU.1 in circulating monocytes.
Keywords: Human; inflammation; monocytes/macrophages; mucosa.
© 2017 The Authors. Immunity, Inflammation and Disease Published by John Wiley & Sons Ltd.
Figures
References
-
- Hooper, L. V. , and Macpherson A. J.. 2010. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10:159–169. - PubMed
-
- Qiao, L. , Braunstein J., Golling M., Schurmann G., Autschbach F., Moller P., and Meuer S.. 1996. Differential regulation of human T cell responsiveness by mucosal versus blood monocytes. Eur. J. Immunol. 26:922–927. - PubMed
-
- Medzhitov, R. 2008. Origin and physiological roles of inflammation. Nature 454:428–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

