The COMET Handbook: version 1.0
- PMID: 28681707
- PMCID: PMC5499094
- DOI: 10.1186/s13063-017-1978-4
The COMET Handbook: version 1.0
Abstract
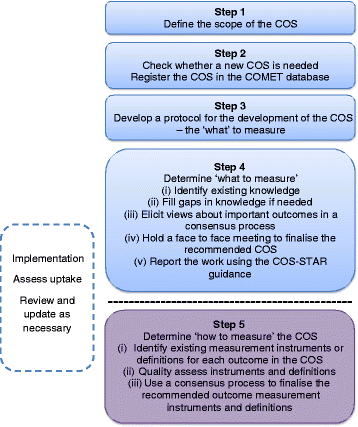
The selection of appropriate outcomes is crucial when designing clinical trials in order to compare the effects of different interventions directly. For the findings to influence policy and practice, the outcomes need to be relevant and important to key stakeholders including patients and the public, health care professionals and others making decisions about health care. It is now widely acknowledged that insufficient attention has been paid to the choice of outcomes measured in clinical trials. Researchers are increasingly addressing this issue through the development and use of a core outcome set, an agreed standardised collection of outcomes which should be measured and reported, as a minimum, in all trials for a specific clinical area.Accumulating work in this area has identified the need for guidance on the development, implementation, evaluation and updating of core outcome sets. This Handbook, developed by the COMET Initiative, brings together current thinking and methodological research regarding those issues. We recommend a four-step process to develop a core outcome set. The aim is to update the contents of the Handbook as further research is identified.
Keywords: COMET Initiative; Clinical trial; Core outcome set; Patients and the public.
Figures

References
-
- Gartlehner G, et al. Criteria for Distinguishing Effectiveness From Efficacy Trials in Systematic Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. (Technical Reviews, No. 12.) - PubMed
-
- Meinert CL. Clinical trials – design, conduct and analysis. 2. New York: Oxford University Press; 2012.
-
- Sackett D, et al. Evidence-Based Medicine: How to Practice and Teach EBM. Edinburgh: Churchill Livingstone; 1997.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

