A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy
- PMID: 28682469
- PMCID: PMC5756520
- DOI: 10.1002/med.21456
A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy
Abstract
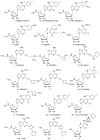
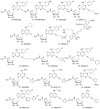
The A3 adenosine receptor (A3 AR) subtype is a novel, promising therapeutic target for inflammatory diseases, such as rheumatoid arthritis (RA) and psoriasis, as well as liver cancer. A3 AR is coupled to inhibition of adenylyl cyclase and regulation of mitogen-activated protein kinase (MAPK) pathways, leading to modulation of transcription. Furthermore, A3 AR affects functions of almost all immune cells and the proliferation of cancer cells. Numerous A3 AR agonists, partial agonists, antagonists, and allosteric modulators have been reported, and their structure-activity relationships (SARs) have been studied culminating in the development of potent and selective molecules with drug-like characteristics. The efficacy of nucleoside agonists may be suppressed to produce antagonists, by structural modification of the ribose moiety. Diverse classes of heterocycles have been discovered as selective A3 AR blockers, although with large species differences. Thus, as a result of intense basic research efforts, the outlook for development of A3 AR modulators for human therapeutics is encouraging. Two prototypical selective agonists, N6-(3-Iodobenzyl)adenosine-5'-N-methyluronamide (IB-MECA; CF101) and 2-chloro-N6-(3-iodobenzyl)-adenosine-5'-N-methyluronamide (Cl-IB-MECA; CF102), have progressed to advanced clinical trials. They were found safe and well tolerated in all preclinical and human clinical studies and showed promising results, particularly in psoriasis and RA, where the A3 AR is both a promising therapeutic target and a biologically predictive marker, suggesting a personalized medicine approach. Targeting the A3 AR may pave the way for safe and efficacious treatments for patient populations affected by inflammatory diseases, cancer, and other conditions.
Keywords: A3 adenosine receptor; cancer; drug development; inflammation; therapy.
© 2017 Wiley Periodicals, Inc.
Figures







References
-
- Antonioli L, Csóka B, Fornai M, et al. Adenosine and inflammation: What’s new on the horizon? Drug Discov Today. 2014;19:1051–1068. - PubMed
-
- Borea PA, Gessi S, Merighi S, Varani K. Adenosine as a multi-signalling guardian angel in human diseases: When, where and how does it exert its protective effects? Trends Pharmacol Sci. 2016;37:419–434. - PubMed
-
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. - PubMed
-
- Molina-Arcas M, Casado FJ, Pastor-Anglada M. Nucleoside transporter proteins. Curr Vasc Pharmacol. 2009;7:426–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

