Direct and tunable modulation of protein levels in rice and wheat with a synthetic small molecule
- PMID: 28682500
- PMCID: PMC5787845
- DOI: 10.1111/pbi.12787
Direct and tunable modulation of protein levels in rice and wheat with a synthetic small molecule
Abstract
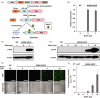
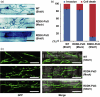
Direct control of protein level enables rapid and efficient analyses of gene functions in crops. Previously, we developed the RDDK-Shield1 (Shld1) system in the model plant Arabidopsis thaliana for direct modulation of protein stabilization using a synthetic small molecule. However, it was unclear whether this system is applicable to economically important crops. In this study, we show that the RDDK-Shld1 system enables rapid and tunable control of protein levels in rice and wheat. Accumulation of RDDK fusion proteins can be reversibly and spatio-temporally controlled by the synthetic small-molecule Shld1. Moreover, RDDK-Bar and RDDK-Pid3 fusions confer herbicide and rice blast resistance, respectively, in a Shld1-dependent manner. Therefore, the RDDK-Shld1 system provides a reversible and tunable technique for controlling protein functions and conditional expression of transgenes in crops.
Keywords: RDDK-Shld1 system; protein stability; rice; small molecule; wheat.
© 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures



Similar articles
-
Molecular Characterization of TaFAR1 Involved in Primary Alcohol Biosynthesis of Cuticular Wax in Hexaploid Wheat.Plant Cell Physiol. 2015 Oct;56(10):1944-61. doi: 10.1093/pcp/pcv112. Epub 2015 Jul 27. Plant Cell Physiol. 2015. PMID: 26220905
-
Characterization of the wheat gene encoding a grain-specific lipid transfer protein TdPR61, and promoter activity in wheat, barley and rice.J Exp Bot. 2012 Mar;63(5):2025-40. doi: 10.1093/jxb/err409. Epub 2012 Jan 2. J Exp Bot. 2012. PMID: 22213809
-
The rice transcription factors OsICE confer enhanced cold tolerance in transgenic Arabidopsis.Plant Signal Behav. 2017 May 4;12(5):e1316442. doi: 10.1080/15592324.2017.1316442. Epub 2017 Apr 17. Plant Signal Behav. 2017. PMID: 28414264 Free PMC article.
-
Functional analyses of TaHMA2, a P(1B)-type ATPase in wheat.Plant Biotechnol J. 2013 May;11(4):420-31. doi: 10.1111/pbi.12027. Epub 2013 Jan 7. Plant Biotechnol J. 2013. PMID: 23294838
-
[Progress in regulation of rice Wx gene expression].Yi Chuan. 2005 Nov;27(6):1013-9. Yi Chuan. 2005. PMID: 16378955 Review. Chinese.
Cited by
-
Manipulation of targeted protein degradation in plant biology.Biochem Soc Trans. 2025 Apr 9;53(2):409-18. doi: 10.1042/BST20230939. Biochem Soc Trans. 2025. PMID: 40209052 Free PMC article. Review.
References
-
- Armstrong, C.M. and Goldberg, D.E. (2007) An FKBP destabilization domain modulates protein levels in Plasmodium falciparum . Nat. Methods, 4, 1007–1009. - PubMed
-
- Azpiroz‐Leehan, R. and Feldmann, K.A. (1997) T‐DNA insertion mutagenesis in Arabidopsis: going back and forth. Trends Genet. 13, 152–156. - PubMed
-
- Bachmair, A. , Finley, D. and Varshavsky, A. (1986) In vivo half‐life of a protein is function of its amino‐terminal residue. Science, 234, 179–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

