Atropine 0.5% eyedrops for the treatment of children with low myopia: A randomized controlled trial
- PMID: 28682887
- PMCID: PMC5502160
- DOI: 10.1097/MD.0000000000007371
Atropine 0.5% eyedrops for the treatment of children with low myopia: A randomized controlled trial
Abstract
Background: This study aimed to assess the efficacy and safety of atropine 0.5% eyedrops (ATE) for the treatment of children with low myopia (LM).
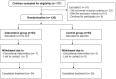
Methods: In this study, a total of 126 children with LM were randomly divided into an intervention group (administered 0.5% ATE) and a control group (administered a placebo), with 63 children in each group. The outcome measurements were changes in the spherical equivalent (SE), and axial length (AL), as well as adverse events (AEs).
Results: Compared with placebo, administration of 0.5% ATE led to less progression in LM, as measured by SE, and less increase in AL (P < .01). In addition, no serious AEs occurred in both the groups.
Conclusion: About 0.5% ATE was efficacious and safe for controlling myopia in children with LM.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Liang CL, Yen E, Su JY, et al. Impact of family history of high myopia on level and onset of myopia. Invest Ophthalmol Vis Sci 2004;45:3446–52. - PubMed
-
- He M, Huang W, Zheng Y, et al. Refractive error and visual impairment in school children in rural southern China. Ophthalmology 2007;114:374–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials