Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models
- PMID: 28683469
- PMCID: PMC5572168
- DOI: 10.1038/bjc.2017.205
Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models
Abstract
Background: Lurbinectedin is a novel anticancer agent currently undergoing late-stage (Phase II /III) clinical evaluation in platinum-resistant ovarian, BRCA1/2-mutated breast and small-cell lung cancer. Lurbinectedin is structurally related to trabectedin and it inhibits active transcription and the DNA repair machinery in tumour cells.
Methods: In this study we investigated whether lurbinectedin has the ability to modulate the inflammatory microenvironment and the viability of myeloid cells in tumour-bearing mice.
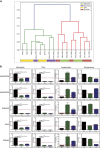
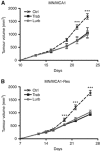
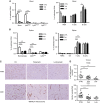
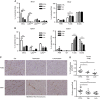
Results: Administration of lurbinectedin significantly and selectively decreased the number of circulating monocytes and, in tumour tissues, that of macrophages and vessels. Similar findings were observed when a lurbinectedin-resistant tumour variant was used, indicating a direct effect of lurbinectedin on the tumour microenviroment. In vitro, lurbinectedin induced caspase-8-dependent apoptosis of human purified monocytes, whereas at low doses it significantly inhibited the production of inflammatory/growth factors (CCL2, CXCL8 and VEGF) and dramatically impaired monocyte adhesion and migration ability. These findings were supported by the strong inhibition of genes of the Rho-GTPase family in lurbinectedin-treated monocytes.
Conclusions: The results illustrate that lurbinectedin affects at multiple levels the inflammatory microenvironment by acting on the viability and functional activity of mononuclear phagocytes. These peculiar effects, combined with its intrinsic activity against cancer cells, make lurbinectedin a compound of particular interest in oncology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ascites interferes with the activity of lurbinectedin and trabectedin: Potential role of their binding to alpha 1-acid glycoprotein.Biochem Pharmacol. 2017 Nov 15;144:52-62. doi: 10.1016/j.bcp.2017.08.001. Epub 2017 Aug 4. Biochem Pharmacol. 2017. PMID: 28782526
-
Comparison of in vitro and in vivo biological effects of trabectedin, lurbinectedin (PM01183) and Zalypsis® (PM00104).Int J Cancer. 2013 Nov;133(9):2024-33. doi: 10.1002/ijc.28213. Epub 2013 May 25. Int J Cancer. 2013. PMID: 23588839
-
Role of macrophage targeting in the antitumor activity of trabectedin.Cancer Cell. 2013 Feb 11;23(2):249-62. doi: 10.1016/j.ccr.2013.01.008. Cancer Cell. 2013. PMID: 23410977
-
Biology of ovarian cancer and trabectedin mechanism of action.Future Oncol. 2013 Dec;9(12 Suppl):11-7. doi: 10.2217/fon.13.199. Future Oncol. 2013. PMID: 24195525 Review.
-
A Comprehensive Review on the Role of Lurbinectedin in Soft Tissue Sarcomas.Curr Treat Options Oncol. 2024 Feb;25(2):176-190. doi: 10.1007/s11864-024-01178-4. Epub 2024 Feb 7. Curr Treat Options Oncol. 2024. PMID: 38324075 Review.
Cited by
-
Novel Cytotoxic Chemotherapies in Small Cell Lung Carcinoma.Cancers (Basel). 2021 Mar 8;13(5):1152. doi: 10.3390/cancers13051152. Cancers (Basel). 2021. PMID: 33800236 Free PMC article. Review.
-
Trabectedin Reveals a Strategy of Immunomodulation in Chronic Lymphocytic Leukemia.Cancer Immunol Res. 2019 Dec;7(12):2036-2051. doi: 10.1158/2326-6066.CIR-19-0152. Epub 2019 Sep 17. Cancer Immunol Res. 2019. PMID: 31530560 Free PMC article.
-
Immunoregulatory effects of Lurbinectedin in chronic lymphocytic leukemia.Cancer Immunol Immunother. 2020 May;69(5):813-824. doi: 10.1007/s00262-020-02513-y. Epub 2020 Feb 13. Cancer Immunol Immunother. 2020. PMID: 32055920 Free PMC article.
-
Development of Marine-Derived Compounds for Cancer Therapy.Mar Drugs. 2021 Jun 15;19(6):342. doi: 10.3390/md19060342. Mar Drugs. 2021. PMID: 34203870 Free PMC article. Review.
-
Navigating the Complexity of Resistance in Lung Cancer Therapy: Mechanisms, Organoid Models, and Strategies for Overcoming Treatment Failure.Cancers (Basel). 2024 Nov 28;16(23):3996. doi: 10.3390/cancers16233996. Cancers (Basel). 2024. PMID: 39682183 Free PMC article. Review.
References
-
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57(1): 289–300.
-
- Breitling R, Armengaud P, Amtmann A, Herzyk P (2004) Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573(1–3): 83–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

