A multi-centre, non-inferiority, randomised controlled trial to compare a cervical pessary with a cervical cerclage in the prevention of preterm delivery in women with short cervical length and a history of preterm birth - PC study
- PMID: 28683739
- PMCID: PMC5501372
- DOI: 10.1186/s12884-017-1393-6
A multi-centre, non-inferiority, randomised controlled trial to compare a cervical pessary with a cervical cerclage in the prevention of preterm delivery in women with short cervical length and a history of preterm birth - PC study
Abstract
Background: Preterm birth is in quantity and in severity the most important contributor of perinatal morbidity and mortality both in well- and low-resource countries. Cervical pessary and cervical cerclage are both considered as preventive treatments in women at risk for preterm birth. We aim to evaluate whether a cervical pessary can replace cervical cerclage for preventing recurrent preterm birth in women with a prior preterm birth due to cervical insufficiency or in women with a prior preterm birth and a short cervix in the current pregnancy.
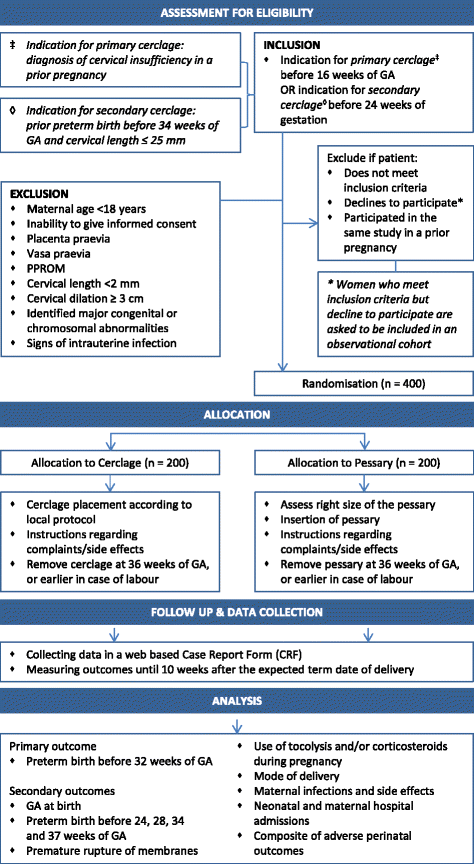
Methods/design: A nationwide open-label multicentre randomised clinical trial will be set up to study women with a singleton pregnancy and a prior preterm birth before 34 weeks of gestation. Women are eligible in case of previous preterm birth based on cervical insufficiency (primary intervention, <16 weeks) or in case of previous preterm birth and a short cervical length in current pregnancy ≤25 mm (secondary intervention, <24 weeks). Eligible women will be randomised to either cervical pessary or cervical cerclage. Both interventions will be removed at labour or at 36 weeks of gestational age, whatever comes first. The primary outcome will be delivery before 32 weeks. Secondary outcomes will be gestational age at birth, preterm birth rate before 24, 28, 34 and 37 weeks of gestation (overall and stratified by spontaneous or indicated delivery), premature rupture of membranes, use of tocolysis and/or corticosteroids during pregnancy, mode of delivery, maternal infections, maternal side effects, neonatal and maternal hospital admissions, and a composite of adverse perinatal outcomes including both morbidity and mortality. We assume an event rate of 20% preterm birth before 32 weeks for cerclage and use a non-inferiority margin of 10% for the cervical pessary. Using an alpha of 0.05 and power of 0.80 we need 2 groups of 200 women each.
Discussion: The outcome of this study will indicate the effectiveness and the cost-effectiveness of a cervical cerclage and of a cervical pessary.
Trial registration:
Netherlands Trial Registry,
Electronic supplementary material: The online version of this article (doi:10.1186/s12884-017-1393-6) contains supplementary material, which is available to authorized users.
Keywords: Cerclage; Morbidity; Pessary; Preterm birth; Prevention.
Conflict of interest statement
Ethics approval and consent to participate
The trial has been approved by the medical ethics committee of the Academic Medical Centre Amsterdam, reference number MEC AMC 2013_365, and by the boards of management of all participating hospitals. The trial is registered in the Netherlands Trial Registry, reference number NTR 4415:
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Esplin MS, O'Brien E, Fraser A, Kerber RA, Clark E, Simonsen SE, et al. Estimating recurrence of spontaneous preterm delivery. Obstet Gynecol. 2008;112(3):516–23. - PubMed
-
- Iams JD, Goldenberg RL, Mercer BM, Moawad A, Thom E, Meis PJ, et al. The preterm prediction study: recurrence risk of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178(5):1035–40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources