Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study
- PMID: 28683766
- PMCID: PMC5500972
- DOI: 10.1186/s13045-017-0501-4
Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study
Abstract
Background: The China Continuation study was a separate regional expansion of the global, double-blind, placebo-controlled, randomized phase III TOURMALINE-MM1 study of ixazomib plus lenalidomide-dexamethasone (Rd) in patients with relapsed/refractory multiple myeloma (RRMM) following one to three prior therapies.
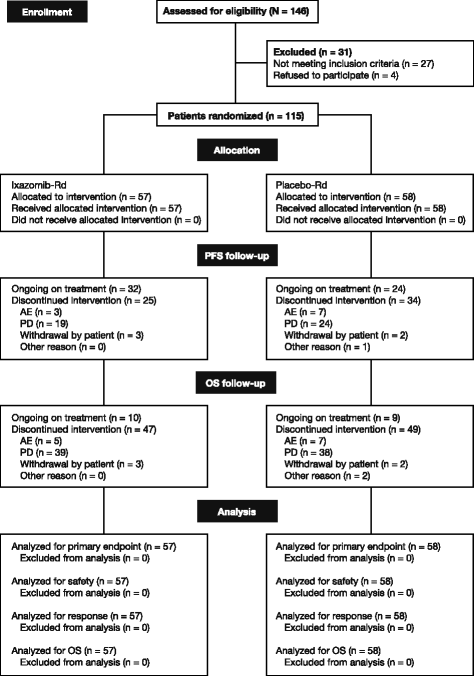
Methods: Patients were randomized (1:1) to receive ixazomib 4.0 mg or placebo on days 1, 8, and 15, plus lenalidomide 25 mg on days 1-21 and dexamethasone 40 mg on days 1, 8, 15, and 22, in 28-day cycles. Randomization was stratified according to number of prior therapies, disease stage, and prior proteasome inhibitor exposure. The primary endpoint was progression-free survival (PFS). In total, 115 Chinese patients were randomized (57 ixazomib-Rd, 58 placebo-Rd).
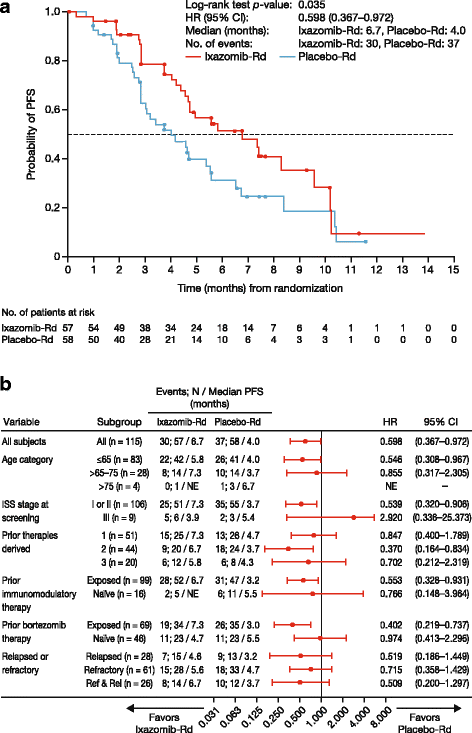
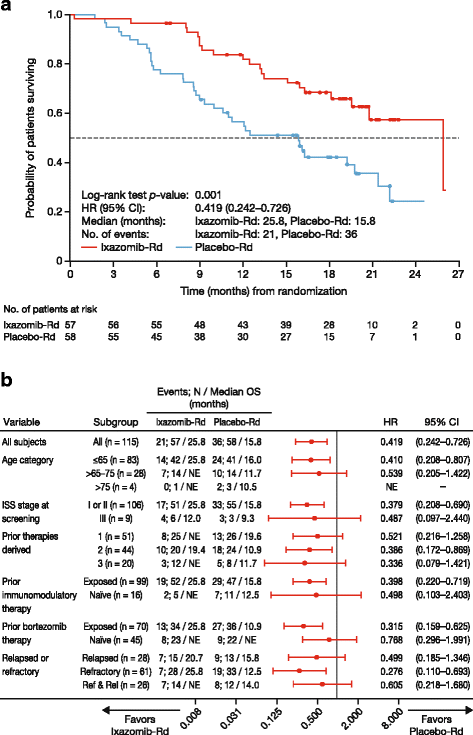
Results: At the preplanned final analysis for PFS, after median PFS follow-up of 7.4 and 6.9 months, respectively, PFS was improved with ixazomib-Rd versus placebo-Rd (median 6.7 vs 4.0 months; HR 0.598; p = 0.035). At the preplanned final analysis of overall survival (OS), after median follow-up of 20.2 and 19.1 months, respectively, OS was improved with ixazomib-Rd versus placebo-Rd (median 25.8 vs 15.8 months; HR 0.419; p = 0.001). On the ixazomib-Rd and placebo-Rd arms, respectively, 38 (67%) and 43 (74%) patients reported grade ≥3 adverse events (AEs), 19 (33%) and 18 (31%) reported serious AEs, and 4 (7%) and 5 (9%) died on-study. The most frequent grade 3/4 AEs were thrombocytopenia (18%/7% vs 14%/5%), neutropenia (19%/5% vs 19%/2%), and anemia (12%/0 vs 26%/2%).
Conclusions: This study demonstrated that PFS and OS were significantly improved with ixazomib-Rd versus placebo-Rd, with limited additional toxicity, in patients with RRMM.
Trial registration: ClinicalTrials.gov, NCT01564537.
Keywords: China; Ixazomib; Multiple myeloma; Oral; Overall survival; Progression-free survival; Proteasome inhibitor; Relapsed/refractory.
Conflict of interest statement
Ethics approval and consent to participate
Details of all sites’ ethics committee approvals are provided in Additional file 3. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
NG, MJH, HL, ZH, BW, XZ, HW, HvdV: employment, Millennium Pharmaceuticals, Inc., Cambridge, MA, USA a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. PGR: consultancy/advisory role for Millennium/Takeda and Celgene. PM: advisory boards for Takeda, Celgene, Janssen, Amgen, and Novartis. JH, JJ, YX, DW, XK, ZD, JLu, XD, XC, JLi, JLiu: no conflicts of interest disclosed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Healthcare resource utilization with ixazomib or placebo plus lenalidomide-dexamethasone in the randomized, double-blind, phase 3 TOURMALINE-MM1 study in relapsed/refractory multiple myeloma.J Med Econ. 2018 Aug;21(8):793-798. doi: 10.1080/13696998.2018.1474745. Epub 2018 May 29. J Med Econ. 2018. PMID: 29741409 Clinical Trial.
-
Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma.N Engl J Med. 2016 Apr 28;374(17):1621-34. doi: 10.1056/NEJMoa1516282. N Engl J Med. 2016. PMID: 27119237 Clinical Trial.
-
Impact of prior therapy on the efficacy and safety of oral ixazomib-lenalidomide-dexamethasone vs. placebo-lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in TOURMALINE-MM1.Haematologica. 2017 Oct;102(10):1767-1775. doi: 10.3324/haematol.2017.170118. Epub 2017 Jul 27. Haematologica. 2017. PMID: 28751562 Free PMC article. Clinical Trial.
-
Ixazomib-lenalidomid-dexamethasone (IRd) in relapsed refractory multiple myeloma (RRMM)-multicenter real-world analysis from Germany and comparative review of the literature.Ann Hematol. 2025 Jul;104(7):3713-3722. doi: 10.1007/s00277-025-06441-8. Epub 2025 Jun 5. Ann Hematol. 2025. PMID: 40471328 Free PMC article. Review.
-
Ixazomib: A Review in Relapsed and/or Refractory Multiple Myeloma.Target Oncol. 2017 Aug;12(4):535-542. doi: 10.1007/s11523-017-0504-7. Target Oncol. 2017. PMID: 28660423 Review.
Cited by
-
Approach to Contemporary Risk Assessment, Prevention and Management of Thrombotic Complications in Multiple Myeloma.Cancers (Basel). 2022 Dec 16;14(24):6216. doi: 10.3390/cancers14246216. Cancers (Basel). 2022. PMID: 36551701 Free PMC article. Review.
-
Efficacy of ixazomib for the treatment of relapsed/refractory multiple myeloma: A protocol of systematic review and meta-analysis.Medicine (Baltimore). 2020 May;99(20):e20211. doi: 10.1097/MD.0000000000020211. Medicine (Baltimore). 2020. PMID: 32443346 Free PMC article.
-
Real-world effectiveness of ixazomib, lenalidomide and dexamethasone in Asians with relapsed/refractory multiple myeloma.Int J Hematol. 2025 May;121(5):670-683. doi: 10.1007/s12185-025-03927-z. Epub 2025 Mar 20. Int J Hematol. 2025. PMID: 40111640 Free PMC article.
-
Re: Arcuri and Americo "Treatment of relapsed/refractory multiple myeloma in the bortezomib and lenalidomide era: a systematic review and network meta-analysis".Ann Hematol. 2022 Jul;101(7):1599-1601. doi: 10.1007/s00277-022-04792-0. Epub 2022 Feb 18. Ann Hematol. 2022. PMID: 35179641 Free PMC article. No abstract available.
-
Clinical Pharmacology of Ixazomib: The First Oral Proteasome Inhibitor.Clin Pharmacokinet. 2019 Apr;58(4):431-449. doi: 10.1007/s40262-018-0702-1. Clin Pharmacokinet. 2019. PMID: 30117017 Free PMC article. Review.
References
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda: National Cancer Institute; 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

