Patient preferences for dry powder inhaler attributes in asthma and chronic obstructive pulmonary disease in France: a discrete choice experiment
- PMID: 28683805
- PMCID: PMC5501405
- DOI: 10.1186/s12890-017-0439-x
Patient preferences for dry powder inhaler attributes in asthma and chronic obstructive pulmonary disease in France: a discrete choice experiment
Abstract
Background: Dry powder inhalers (DPIs) are often used in asthma and chronic obstructive pulmonary disease (COPD) therapies. Using the discrete choice experiment (DCE) methodology, this study conducted in France was designed to assess patients' preferences for different attributes of DPIs.
Methods: Attributes of DPIs were defined based on a literature review, patient focus group discussions and interviews with healthcare professionals (qualitative phase of the study). An online survey was then conducted among French patients with asthma or COPD to elicit patient preferences and willingness to pay (WTP) for these attributes using the DCE methodology (quantitative phase). A fractional factorial design including three blocks of 12 choice sets was created. Each choice set comprised three alternatives: two fictitious inhalers and the patient's current inhaler. Marginal utilities were estimated using a ranked ordered logit model. Interactions between attributes and disease (asthma or COPD) were tested.
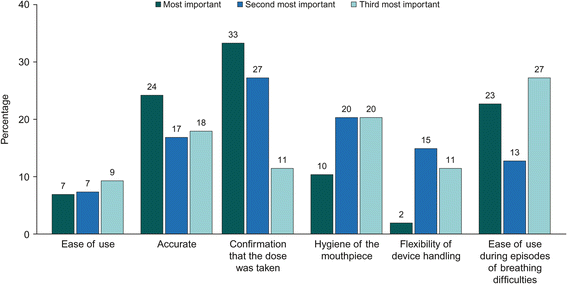
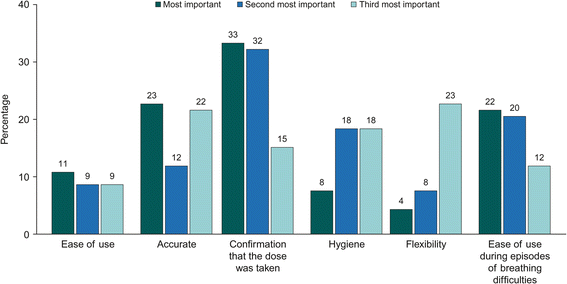
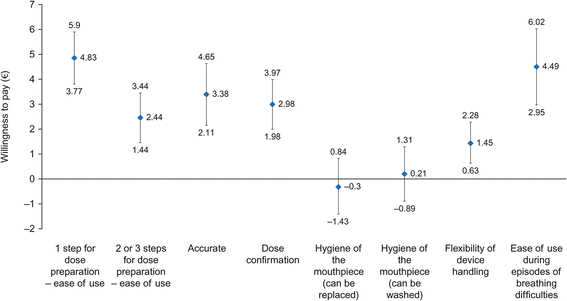
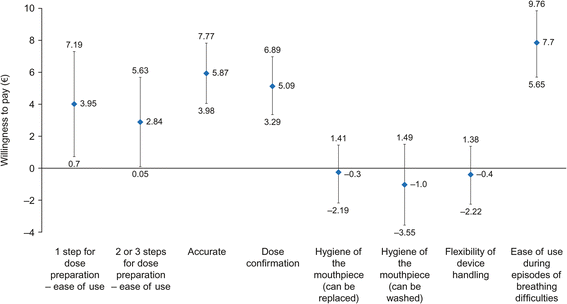
Results: Six DPI attributes were defined based on the qualitative phase: ease of use/fool-proof priming; accurate and easy-to-read dose counter; dose confirmation; hygiene of the mouthpiece; flexibility of the device handling; ability to use the inhaler with breathing difficulties. Overall, 201 patients with asthma and 93 with COPD were included in the online survey. Patients with asthma placed most value on an inhaler that requires one step for dose preparation (WTP €4.83 [95% CI: €3.77-€5.90], relative to an inhaler requiring four steps) and one that could be used during episodes of breathing difficulties (WTP €4.49 [95% CI: €2.95-€6.02]). Patients with COPD placed most value on an inhaler that could be used during episodes of breathing difficulties (WTP €7.70 [95% CI: €5.65-€9.76]) and on the accuracy of the dose counter (WTP €5.87 [95% CI: €3.98-€ 7.77]).
Conclusion: This study suggests that asthma and COPD patients would be willing to change their inhaler if they were offered the option of a new inhaler with improved characteristics and they place a high value on an inhaler with ease of use during breathing difficulty episodes.
Keywords: Asthma; COPD; Discrete choice experiment; Patient preference; Willingness to pay.
Conflict of interest statement
Consent for publication
Not applicable.
Competing interests
NH, MEN, IA, MT and SA are employees of Creativ-Ceutical; ST and AP are employees of Teva Pharmaceuticals.
NR has received fees for participating in advisory boards, presenting in scientific symposia, consulting, participating in medical congresses from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Mundipharma, Novartis, Pfizer, Sandoz, Sanofi, 3M Pharmaceutical, and Teva Pharmaceuticals; research funding from Boehringer Ingelheim, Novartis, and Pfizer; and was a Study Coordinator in research funded by Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment.Thorax. 2020 Sep;75(9):735-743. doi: 10.1136/thoraxjnl-2019-213974. Epub 2020 Jul 6. Thorax. 2020. PMID: 32631932 Free PMC article.
-
Qualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPD.BMC Pulm Med. 2013 Dec 7;13:72. doi: 10.1186/1471-2466-13-72. BMC Pulm Med. 2013. PMID: 24314123 Free PMC article. Clinical Trial.
-
Patient preference for chronic obstructive pulmonary disease (COPD) treatment inhalers: a discrete choice experiment in France.Curr Med Res Opin. 2019 May;35(5):785-792. doi: 10.1080/03007995.2019.1574507. Epub 2019 Feb 15. Curr Med Res Opin. 2019. PMID: 30681007
-
Dry powder inhalers - between the doctor and the patient.Adv Respir Med. 2018;86(1):44-52. doi: 10.5603/ARM.2017.0061. Adv Respir Med. 2018. PMID: 29490421 Review.
-
Understanding Dry Powder Inhalers: Key Technical and Patient Preference Attributes.Adv Ther. 2019 Oct;36(10):2547-2557. doi: 10.1007/s12325-019-01066-6. Epub 2019 Sep 2. Adv Ther. 2019. PMID: 31478131 Free PMC article. Review.
Cited by
-
Incorporating the Environmental Impact into a Budget Impact Analysis: The Example of Adopting RESPIMAT® Re-usable Inhaler.Appl Health Econ Health Policy. 2020 Jun;18(3):433-442. doi: 10.1007/s40258-019-00540-0. Appl Health Econ Health Policy. 2020. PMID: 31808066 Free PMC article.
-
A Budget Impact Model to Estimate the Environmental Impact of Adopting RESPIMAT® Re-usable in the Nordics and Benelux.Adv Ther. 2019 Dec;36(12):3435-3445. doi: 10.1007/s12325-019-01114-1. Epub 2019 Oct 17. Adv Ther. 2019. PMID: 31625130 Free PMC article.
-
Preferences of patients with asthma or COPD for treatments in pulmonary rehabilitation.Health Econ Rev. 2021 Apr 17;11(1):14. doi: 10.1186/s13561-021-00308-0. Health Econ Rev. 2021. PMID: 33866476 Free PMC article.
-
Comparison of Two pMDIs in Adult Asthmatics: A Randomized Double-Blind Double-Dummy Clinical Trial.Tuberc Respir Dis (Seoul). 2022 Jan;85(1):25-36. doi: 10.4046/trd.2021.0093. Epub 2021 Nov 29. Tuberc Respir Dis (Seoul). 2022. PMID: 34839622 Free PMC article.
-
A Systematic and Critical Review of Discrete Choice Experiments in Asthma and Chronic Obstructive Pulmonary Disease.Patient. 2022 Jan;15(1):55-68. doi: 10.1007/s40271-021-00536-w. Epub 2021 Jul 12. Patient. 2022. PMID: 34250574 Free PMC article.
References
-
- Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51(2):158–72. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

