Mechano growth factor, a splice variant of IGF-1, promotes neurogenesis in the aging mouse brain
- PMID: 28683812
- PMCID: PMC5501366
- DOI: 10.1186/s13041-017-0304-0
Mechano growth factor, a splice variant of IGF-1, promotes neurogenesis in the aging mouse brain
Abstract
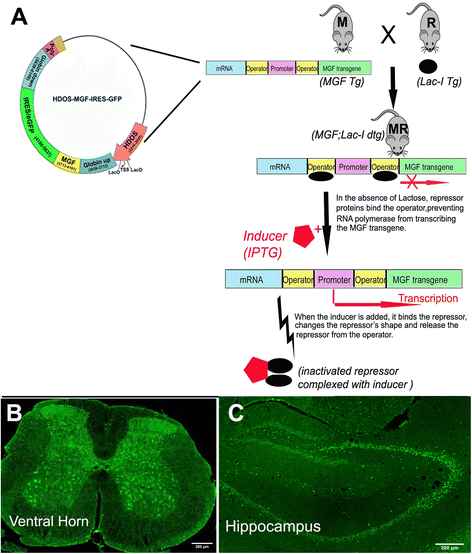
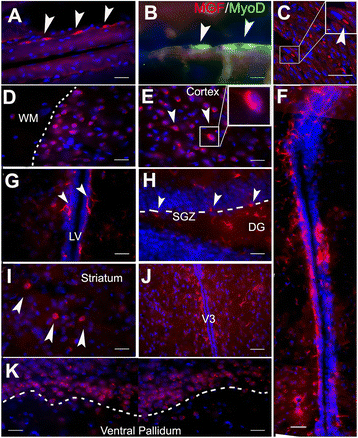
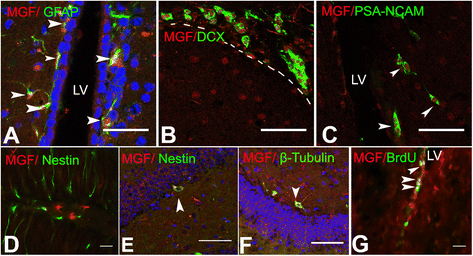
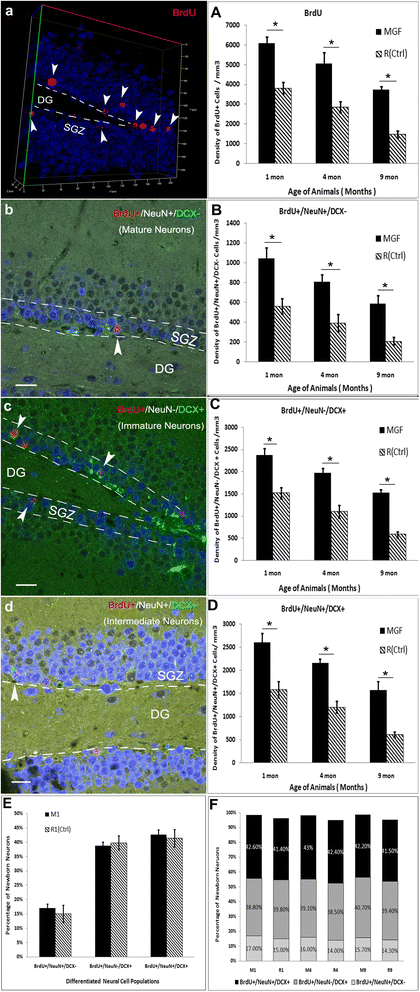
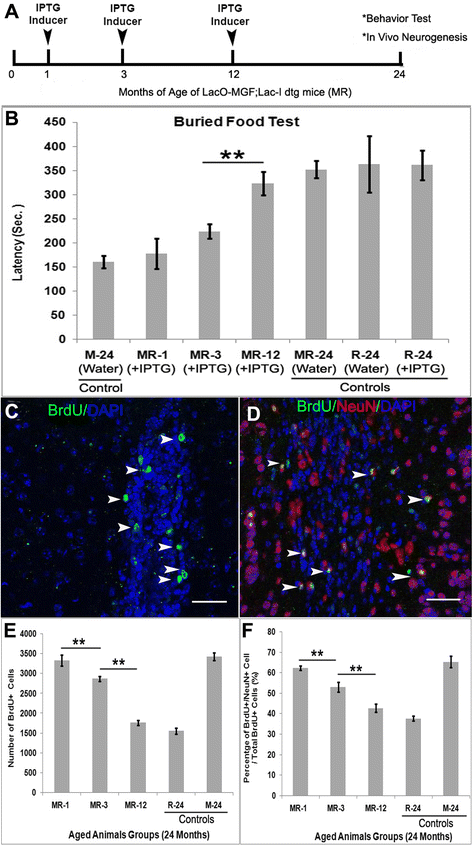
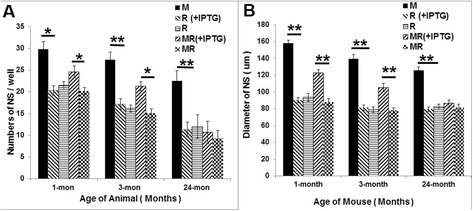
Mechano growth factor (MGF) is a splice variant of IGF-1 first described in skeletal muscle. MGF induces muscle cell proliferation in response to muscle stress and injury. In control mice we found endogenous expression of MGF in neurogenic areas of the brain and these levels declined with age. To better understand the role of MGF in the brain, we used transgenic mice that constitutively overexpressed MGF from birth. MGF overexpression significantly increased the number of BrdU+ proliferative cells in the dentate gyrus (DG) of the hippocampus and subventricular zone (SVG). Although MGF overexpression increased the overall rate of adult hippocampal neurogenesis at the proliferation stage it did not alter the distribution of neurons at post-mitotic maturation stages. We then used the lac-operon system to conditionally overexpress MGF in the mouse brain beginning at 1, 3 and 12 months with histological and behavioral observation at 24 months of age. With conditional overexpression there was an increase of BrdU+ proliferating cells and BrdU+ differentiated mature neurons in the olfactory bulbs at 24 months when overexpression was induced from 1 and 3 months of age but not when started at 12 months. This was associated with preserved olfactory function. In vitro, MGF increased the size and number of neurospheres harvested from SVZ-derived neural stem cells (NSCs). These findings indicate that MGF overexpression increases the number of neural progenitor cells and promotes neurogenesis but does not alter the distribution of adult newborn neurons at post-mitotic stages. Maintaining youthful levels of MGF may be important in reversing age-related neuronal loss and brain dysfunction.
Keywords: Aging; Insulin-like growth factor-I (IGF-I); Mechano growth factor (MGF); Neural stem cells; Neurogenesis; Neuroprotection.
Figures
Similar articles
-
In vitro investigation of growth factors including MGF and IGF-1 in neural stem cell activation, proliferation, and migration.Brain Res. 2021 May 15;1759:147366. doi: 10.1016/j.brainres.2021.147366. Epub 2021 Feb 16. Brain Res. 2021. PMID: 33607046
-
Brain Insulin-Like Growth Factor-I Directs the Transition from Stem Cells to Mature Neurons During Postnatal/Adult Hippocampal Neurogenesis.Stem Cells. 2016 Aug;34(8):2194-209. doi: 10.1002/stem.2397. Epub 2016 May 27. Stem Cells. 2016. PMID: 27144663
-
Phosphodiesterase7 Inhibition Activates Adult Neurogenesis in Hippocampus and Subventricular Zone In Vitro and In Vivo.Stem Cells. 2017 Feb;35(2):458-472. doi: 10.1002/stem.2480. Epub 2016 Sep 16. Stem Cells. 2017. PMID: 27538853
-
Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration.Endocrinology. 2010 Mar;151(3):865-75. doi: 10.1210/en.2009-1217. Epub 2010 Feb 3. Endocrinology. 2010. PMID: 20130113 Free PMC article. Review.
-
Insulin-like growth factor-I and neurogenesis in the adult mammalian brain.Brain Res Dev Brain Res. 2002 Mar 31;134(1-2):115-22. doi: 10.1016/s0165-3806(02)00277-8. Brain Res Dev Brain Res. 2002. PMID: 11947942 Review.
Cited by
-
Role of Alternatively Spliced Messenger RNA (mRNA) Isoforms of the Insulin-Like Growth Factor 1 (IGF1) in Selected Human Tumors.Int J Mol Sci. 2020 Sep 23;21(19):6995. doi: 10.3390/ijms21196995. Int J Mol Sci. 2020. PMID: 32977489 Free PMC article. Review.
-
Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice.Geroscience. 2019 Apr;41(2):185-208. doi: 10.1007/s11357-019-00065-3. Epub 2019 May 10. Geroscience. 2019. PMID: 31076997 Free PMC article.
-
The Effects of IGF1 and MGF on Neural Stem Cells in Hypoxic Conditions.Basic Clin Neurosci. 2024 May-Jun;15(3):343-354. doi: 10.32598/bcn.2022.3981.1. Epub 2024 May 1. Basic Clin Neurosci. 2024. PMID: 39403354 Free PMC article.
-
Nutrient-Response Pathways in Healthspan and Lifespan Regulation.Cells. 2022 May 6;11(9):1568. doi: 10.3390/cells11091568. Cells. 2022. PMID: 35563873 Free PMC article. Review.
-
Age‑dependent decreases in insulin‑like growth factor‑I and its receptor expressions in the gerbil olfactory bulb.Mol Med Rep. 2018 Jun;17(6):8161-8166. doi: 10.3892/mmr.2018.8886. Epub 2018 Apr 13. Mol Med Rep. 2018. PMID: 29658594 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

