Sortase Transpeptidases: Structural Biology and Catalytic Mechanism
- PMID: 28683919
- PMCID: PMC5718342
- DOI: 10.1016/bs.apcsb.2017.04.008
Sortase Transpeptidases: Structural Biology and Catalytic Mechanism
Abstract
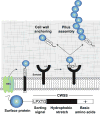
Gram-positive bacteria use sortase cysteine transpeptidase enzymes to covalently attach proteins to their cell wall and to assemble pili. In pathogenic bacteria sortases are potential drug targets, as many of the proteins that they display on the microbial surface play key roles in the infection process. Moreover, the Staphylococcus aureus Sortase A (SaSrtA) enzyme has been developed into a valuable biochemical reagent because of its ability to ligate biomolecules together in vitro via a covalent peptide bond. Here we review what is known about the structures and catalytic mechanism of sortase enzymes. Based on their primary sequences, most sortase homologs can be classified into six distinct subfamilies, called class A-F enzymes. Atomic structures reveal unique, class-specific variations that support alternate substrate specificities, while structures of sortase enzymes bound to sorting signal mimics shed light onto the molecular basis of substrate recognition. The results of computational studies are reviewed that provide insight into how key reaction intermediates are stabilized during catalysis, as well as the mechanism and dynamics of substrate recognition. Lastly, the reported in vitro activities of sortases are compared, revealing that the transpeptidation activity of SaSrtA is at least 20-fold faster than other sortases that have thus far been characterized. Together, the results of the structural, computational, and biochemical studies discussed in this review begin to reveal how sortases decorate the microbial surface with proteins and pili, and may facilitate ongoing efforts to discover therapeutically useful small molecule inhibitors.
Keywords: Mechanism; Sortase; Structure; Transpeptidase.
© 2017 Elsevier Inc. All rights reserved.
Figures





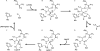
References
-
- Aulabaugh A, Ding W, Kapoor B, Tabei K, Alksne L, Dushin R, et al. Development of an HPLC assay for Staphylococcus aureus sortase: Evidence for the formation of the kinetically competent acyl enzyme intermediate. Analytical Biochemistry. 2007;360(1):14–22. - PubMed
-
- Bentley ML, Gaweska H, Kielec JM, McCafferty DG. Engineering the substrate specificity of Staphylococcus aureus Sortase A. The beta6/beta7 loop from SrtB confers NPQTN recognition to SrtA. The Journal of Biological Chemistry. 2007;282(9):6571–6581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

