Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients With Breast Cancer
- PMID: 28683962
- PMCID: PMC5665653
- DOI: 10.1016/j.jacc.2017.05.019
Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients With Breast Cancer
Erratum in
-
Correction.J Am Coll Cardiol. 2017 Nov 28;70(21):2738. doi: 10.1016/j.jacc.2017.10.041. J Am Coll Cardiol. 2017. PMID: 29169492 No abstract available.
Abstract
Background: Oxidative/nitrosative stress and endothelial dysfunction are hypothesized to be central to cancer therapeutics-related cardiac dysfunction (CTRCD). However, the relationship between circulating arginine-nitric oxide (NO) metabolites and CTRCD remains unstudied.
Objectives: This study sought to examine the relationship between arginine-NO metabolites and CTRCD in a prospective cohort of 170 breast cancer patients treated with doxorubicin with or without trastuzumab.
Methods: Plasma levels of arginine, citrulline, ornithine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and N-monomethylarginine (MMA) were quantified at baseline, 1 month, and 2 months after doxorubicin initiation. Determinants of baseline biomarker levels were identified using multivariable linear regression, and Cox regression defined the association between baseline levels and 1- or 2-month biomarker changes and CTRCD rate in 139 participants with quantitated echocardiograms at all time points.
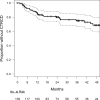
Results: Age, hypertension, body mass index, and African-American race were independently associated with ≥1 of baseline citrulline, ADMA, SDMA, and MMA levels. Decreases in arginine and citrulline and increases in ADMA were observed at 1 and 2 months (all p < 0.05). Overall, 32 participants experienced CTRCD over a maximum follow-up of 5.4 years. Hazard ratios for ADMA and MMA at 2 months were 3.33 (95% confidence interval [CI]: 1.12 to 9.96) and 2.70 (95% CI: 1.35 to 5.41), respectively, and 0.78 (95% CI: 0.64 to 0.97) for arginine at 1 month.
Conclusions: In breast cancer patients undergoing doxorubicin therapy, early alterations in arginine-NO metabolite levels occurred, and early biomarker changes were associated with a greater CTRCD rate. Our findings highlight the potential mechanistic and translational relevance of this pathway to CTRCD.
Keywords: arginine metabolism; cardio-oncology; cardiotoxicity; doxorubicin; nitrosative stress; trastuzumab.
Copyright © 2017 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
The Horizon in Cardio-Oncology: "You Are Only as Good as Your Endothelium".J Am Coll Cardiol. 2017 Jul 11;70(2):163-164. doi: 10.1016/j.jacc.2017.05.048. J Am Coll Cardiol. 2017. PMID: 28683963 No abstract available.
-
Statistical Analyses of Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction.J Am Coll Cardiol. 2017 Dec 19;70(24):3071. doi: 10.1016/j.jacc.2017.09.1137. J Am Coll Cardiol. 2017. PMID: 29241495 No abstract available.
-
Flow-Mediated Dilation of Brachial Artery as a Screening Tool for Anthracycline-Induced Cardiotoxicity.J Am Coll Cardiol. 2017 Dec 19;70(24):3072. doi: 10.1016/j.jacc.2017.09.1140. J Am Coll Cardiol. 2017. PMID: 29241496 No abstract available.
-
Reply: Statistical Analyses of Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction.J Am Coll Cardiol. 2017 Dec 19;70(24):3073. doi: 10.1016/j.jacc.2017.09.1141. J Am Coll Cardiol. 2017. PMID: 29241497 Free PMC article. No abstract available.
References
-
- Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–12. - PubMed
-
- Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol. 2012;30:3657–64. - PubMed
-
- Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Ther. 2000;294:396–401. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

