Non-canonical proteolytic activation of human prothrombin by subtilisin from Bacillus subtilis may shift the procoagulant-anticoagulant equilibrium toward thrombosis
- PMID: 28684417
- PMCID: PMC5602379
- DOI: 10.1074/jbc.M117.795245
Non-canonical proteolytic activation of human prothrombin by subtilisin from Bacillus subtilis may shift the procoagulant-anticoagulant equilibrium toward thrombosis
Abstract
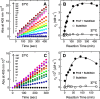
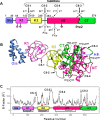
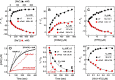
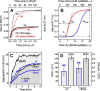
Blood coagulation is a finely regulated physiological process culminating with the factor Xa (FXa)-mediated conversion of the prothrombin (ProT) zymogen to active α-thrombin (αT). In the prothrombinase complex on the platelet surface, FXa cleaves ProT at Arg-271, generating the inactive precursor prethrombin-2 (Pre2), which is further attacked at Arg-320-Ile-321 to yield mature αT. Whereas the mechanism of physiological ProT activation has been elucidated in great detail, little is known about the role of bacterial proteases, possibly released in the bloodstream during infection, in inducing blood coagulation by direct proteolytic ProT activation. This knowledge gap is particularly concerning, as bacterial infections are frequently complicated by severe coagulopathies. Here, we show that addition of subtilisin (50 nm to 2 μm), a serine protease secreted by the non-pathogenic bacterium Bacillus subtilis, induces plasma clotting by proteolytically converting ProT into active σPre2, a nicked Pre2 derivative with a single cleaved Ala-470-Asn-471 bond. Notably, we found that this non-canonical cleavage at Ala-470-Asn-471 is instrumental for the onset of catalysis in σPre2, which was, however, reduced about 100-200-fold compared with αT. Of note, σPre2 could generate fibrin clots from fibrinogen, either in solution or in blood plasma, and could aggregate human platelets, either isolated or in whole blood. Our findings demonstrate that alternative cleavage of ProT by proteases, even by those secreted by non-virulent bacteria such as B. subtilis, can shift the delicate procoagulant-anticoagulant equilibrium toward thrombosis.
Keywords: coagulation factor; infection; proteolysis; prothrombin; serine protease.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures








Similar articles
-
Active site-labeled prothrombin inhibits prothrombinase in vitro and thrombosis in vivo.J Biol Chem. 2011 Jul 1;286(26):23345-56. doi: 10.1074/jbc.M111.230292. Epub 2011 Apr 29. J Biol Chem. 2011. PMID: 21531712 Free PMC article.
-
Expression of allosteric linkage between the sodium ion binding site and exosite I of thrombin during prothrombin activation.J Biol Chem. 2007 Jun 1;282(22):16095-104. doi: 10.1074/jbc.M610577200. Epub 2007 Apr 12. J Biol Chem. 2007. PMID: 17430903 Free PMC article.
-
Specificity and affinity of the N-terminal residues in staphylocoagulase in binding to prothrombin.J Biol Chem. 2020 Apr 24;295(17):5614-5625. doi: 10.1074/jbc.RA120.012588. Epub 2020 Mar 10. J Biol Chem. 2020. PMID: 32156702 Free PMC article.
-
Formation of the fibrin clot: the balance of procoagulant and inhibitory factors.Clin Haematol. 1985 Jun;14(2):281-342. Clin Haematol. 1985. PMID: 2994929 Review.
-
The regulation of clotting factors.Crit Rev Eukaryot Gene Expr. 1997;7(3):241-80. doi: 10.1615/critreveukargeneexpr.v7.i3.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9399073 Review.
Cited by
-
A conserved Neurite Outgrowth and Guidance motif with biomimetic potential in neuronal Cell Adhesion Molecules.Comput Struct Biotechnol J. 2021 Oct 12;19:5622-5636. doi: 10.1016/j.csbj.2021.10.005. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34712402 Free PMC article.
-
Structure of Coagulation Factor II: Molecular Mechanism of Thrombin Generation and Development of Next-Generation Anticoagulants.Front Med (Lausanne). 2018 Oct 2;5:281. doi: 10.3389/fmed.2018.00281. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30333979 Free PMC article. Review.
-
The Role of Proteolysis in Amyloidosis.Int J Mol Sci. 2022 Dec 31;24(1):699. doi: 10.3390/ijms24010699. Int J Mol Sci. 2022. PMID: 36614141 Free PMC article. Review.
-
Proteases: Pivot Points in Functional Proteomics.Methods Mol Biol. 2019;1871:313-392. doi: 10.1007/978-1-4939-8814-3_20. Methods Mol Biol. 2019. PMID: 30276748 Free PMC article. Review.
-
Exogenous human α-Synuclein acts in vitro as a mild platelet antiaggregant inhibiting α-thrombin-induced platelet activation.Sci Rep. 2022 Jun 14;12(1):9880. doi: 10.1038/s41598-022-12886-y. Sci Rep. 2022. PMID: 35701444 Free PMC article.
References
-
- Roberts H. R., Hoffman M., and Monroe D. M. (2006) A cell-based model of thrombin generation. Semin. Thromb. Hemost. 32, 32–38 - PubMed
-
- Versteeg H. H., Heemskerk J. W., Levi M., and Reitsma P. H. (2013) New fundamentals in hemostasis. Physiol. Rev. 93, 327–358 - PubMed
-
- Butenas S., van't Veer C., and Mann K. G. (1999) “Normal” thrombin generation. Blood 94, 2169–2178 - PubMed
-
- Mann K. G., Krishnaswamy S., and Lawson J. H. (1992) Surface-dependent hemostasis. Semin. Hematol. 29, 213–226 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

