Structural and biochemical analyses indicate that a bacterial persulfide dioxygenase-rhodanese fusion protein functions in sulfur assimilation
- PMID: 28684420
- PMCID: PMC5572905
- DOI: 10.1074/jbc.M117.790170
Structural and biochemical analyses indicate that a bacterial persulfide dioxygenase-rhodanese fusion protein functions in sulfur assimilation
Abstract
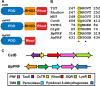
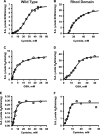
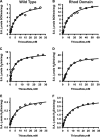
Hydrogen sulfide (H2S) is a signaling molecule that is toxic at elevated concentrations. In eukaryotes, it is cleared via a mitochondrial sulfide oxidation pathway, which comprises sulfide quinone oxidoreductase, persulfide dioxygenase (PDO), rhodanese, and sulfite oxidase and converts H2S to thiosulfate and sulfate. Natural fusions between the non-heme iron containing PDO and rhodanese, a thiol sulfurtransferase, exist in some bacteria. However, little is known about the role of the PDO-rhodanese fusion (PRF) proteins in sulfur metabolism. Herein, we report the kinetic properties and the crystal structure of a PRF from the Gram-negative endophytic bacterium Burkholderia phytofirmans The crystal structures of wild-type PRF and a sulfurtransferase-inactivated C314S mutant with and without glutathione were determined at 1.8, 2.4, and 2.7 Å resolution, respectively. We found that the two active sites are distant and do not show evidence of direct communication. The B. phytofirmans PRF exhibited robust PDO activity and preferentially catalyzed sulfur transfer in the direction of thiosulfate to sulfite and glutathione persulfide; sulfur transfer in the reverse direction was detectable only under limited turnover conditions. Together with the kinetic data, our bioinformatics analysis reveals that B. phytofirmans PRF is poised to metabolize thiosulfate to sulfite in a sulfur assimilation pathway rather than in sulfide stress response as seen, for example, with the Staphylococcus aureus PRF or sulfide oxidation and disposal as observed with the homologous mammalian proteins.
Keywords: X-ray crystallography; enzyme kinetics; hydrogen sulfide; iron; sulfur.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
Thiosulfate sulfurtransferase-like domain-containing 1 protein interacts with thioredoxin.J Biol Chem. 2018 Feb 23;293(8):2675-2686. doi: 10.1074/jbc.RA117.000826. Epub 2018 Jan 18. J Biol Chem. 2018. PMID: 29348167 Free PMC article.
-
Rhodaneses minimize the accumulation of cellular sulfane sulfur to avoid disulfide stress during sulfide oxidation in bacteria.Redox Biol. 2022 Jul;53:102345. doi: 10.1016/j.redox.2022.102345. Epub 2022 May 26. Redox Biol. 2022. PMID: 35653932 Free PMC article.
-
Recombinant Escherichia coli with sulfide:quinone oxidoreductase and persulfide dioxygenase rapidly oxidises sulfide to sulfite and thiosulfate via a new pathway.Environ Microbiol. 2016 Dec;18(12):5123-5136. doi: 10.1111/1462-2920.13511. Epub 2016 Sep 23. Environ Microbiol. 2016. PMID: 27573649
-
Essential role of sulfide oxidation in brain health and neurological disorders.Pharmacol Ther. 2025 Feb;266:108787. doi: 10.1016/j.pharmthera.2024.108787. Epub 2024 Dec 22. Pharmacol Ther. 2025. PMID: 39719173 Review.
-
Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications.Int J Mol Sci. 2022 Jul 30;23(15):8452. doi: 10.3390/ijms23158452. Int J Mol Sci. 2022. PMID: 35955583 Free PMC article. Review.
Cited by
-
Regulation of the redox metabolome and thiol proteome by hydrogen sulfide.Crit Rev Biochem Mol Biol. 2021 Jun;56(3):221-235. doi: 10.1080/10409238.2021.1893641. Epub 2021 Mar 15. Crit Rev Biochem Mol Biol. 2021. PMID: 33722121 Free PMC article.
-
Sensing and regulation of reactive sulfur species (RSS) in bacteria.Curr Opin Chem Biol. 2023 Oct;76:102358. doi: 10.1016/j.cbpa.2023.102358. Epub 2023 Jul 1. Curr Opin Chem Biol. 2023. PMID: 37399745 Free PMC article. Review.
-
Visualizing the superfamily of metallo-β-lactamases through sequence similarity network neighborhood connectivity analysis.Heliyon. 2021 Jan 2;7(1):e05867. doi: 10.1016/j.heliyon.2020.e05867. eCollection 2021 Jan. Heliyon. 2021. PMID: 33426353 Free PMC article.
-
Thiosulfate sulfurtransferase-like domain-containing 1 protein interacts with thioredoxin.J Biol Chem. 2018 Feb 23;293(8):2675-2686. doi: 10.1074/jbc.RA117.000826. Epub 2018 Jan 18. J Biol Chem. 2018. PMID: 29348167 Free PMC article.
-
Thioredoxin regulates human mercaptopyruvate sulfurtransferase at physiologically-relevant concentrations.J Biol Chem. 2020 May 8;295(19):6299-6311. doi: 10.1074/jbc.RA120.012616. Epub 2020 Mar 16. J Biol Chem. 2020. PMID: 32179647 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

