Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force
- PMID: 28684559
- PMCID: PMC5754738
- DOI: 10.1136/annrheumdis-2017-211734
Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force
Erratum in
-
Correction: Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force.Ann Rheum Dis. 2018 Mar;77(3):472. doi: 10.1136/annrheumdis-2017-211734corr1. Ann Rheum Dis. 2018. PMID: 29440019 Free PMC article. No abstract available.
Abstract
Therapeutic targets have been defined for axial and peripheral spondyloarthritis (SpA) in 2012, but the evidence for these recommendations was only of indirect nature. These recommendations were re-evaluated in light of new insights. Based on the results of a systematic literature review and expert opinion, a task force of rheumatologists, dermatologists, patients and a health professional developed an update of the 2012 recommendations. These underwent intensive discussions, on site voting and subsequent anonymous electronic voting on levels of agreement with each item. A set of 5 overarching principles and 11 recommendations were developed and voted on. Some items were present in the previous recommendations, while others were significantly changed or newly formulated. The 2017 task force arrived at a single set of recommendations for axial and peripheral SpA, including psoriatic arthritis (PsA). The most exhaustive discussions related to whether PsA should be assessed using unidimensional composite scores for its different domains or multidimensional scores that comprise multiple domains. This question was not resolved and constitutes an important research agenda. There was broad agreement, now better supported by data than in 2012, that remission/inactive disease and, alternatively, low/minimal disease activity are the principal targets for the treatment of PsA. As instruments to assess the patients on the path to the target, the Ankylosing Spondylitis Disease Activity Score (ASDAS) for axial SpA and the Disease Activity index for PSoriatic Arthritis (DAPSA) and Minimal Disease Activity (MDA) for PsA were recommended, although not supported by all. Shared decision-making between the clinician and the patient was seen as pivotal to the process. The task force defined the treatment target for SpA as remission or low disease activity and developed a large research agenda to further advance the field.
Keywords: Ankylosing Spondylitis; Outcomes Research; Psoriatic Arthritis; Spondyloarthritis; Treatment.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: DA served as a consultant and/or speaker for Abbvie, Astra-Zeneca, BMS, Janssen, Medac, MSD, Pfizer, Roche, UCB and received grant support from BMS. XB has served as consultant and/or speaker for Abbvie, BMS, Celgene, Chugai, Janssen, Novartis, Pfizer, UCB. NB has received consultancy fees for work commissioned by Grünenthal, Lilly, Janssen, PfizerLaura Coates has received research funding from Abbvie and Janssen and honoraria from Abbvie, Amgen, BMS, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer, Sun Pharma, UCBMaxime Dougados has participated as a speaker in symposia or as an advisor in boards organized by Pfizer, Abbvie, Ucb, Merck, Amgen, Novartis, Lilly, Bms, Roche and his department has received research grants from Pfizer, Abbvie, Ucb, Merck, Amgen, Novartis, Lilly, Bms, Roche. Laure Gossec has received honoraria or research funding from Abbvie, BMS, Celgene, Janseen, MSD, Novartis, Pfizer, Roche and UCB. PE has undertaken clinical trials and provided expert advice to Pfizer, MSD, Abbvie, BMS, UCB, Roche, Novartis, Samsung, Sandoz and Lilly. OF reports grants and personal fees from Novartis, personal fees from Pfizer, personal fees from Lilly, personal fees from Cellgene, grants and personal fees from Abbvie, personal fees from Janssen, personal fees from UCB, outside the submitted work. AK has served as consultant and/or performed clinical research for Abbvie, Amgen, Celgene, Janssen, Novartis, UCB. PMM has received consultancy/speaker’s fees from AbbVie, Centocor, Janssen, Merck, Novartis, Pfizer and UCB. AM has received honoraria from Abbvie, MSD and UCBDP has received Grant/research support from: AbbVie, MSD, Novartis, Pfizer, has honoraria/speaker fees from AbbVie, BMS, Boehringer, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, and UCB. MR has received honoraria/ consultancies from Abbvie, BMS, Celgene, Chugai/Roche, Janssen, MSD, Novartis, Pfizer, UCB. JS has received grant support from and/or provided expert advice to Abbvie, Amgen, Astra-Zeneca, BMS, Boehringer-Ingelheim, Celgene, Celltrion, Gilead, Glaxo, Iltoo, Janssen, Lilly, Pfizer, MSD, Roche, Samsung, Novartis-Sandoz, UCB. TS has received honoraria from Abbvie, Janssen, MSD, Novartis and Roche and grant support from Abbvie. FVB has received speaker and/or consultancy fees from AbbVie, Celgene, Janssen,Lilly, MSD, Novartis, Pfizer and UCB. DH has received consulting fees Afrom bbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB, and is Director of Imaging Rheumatology bv. MW has received consulting fees for lectures or advisory board meetings from Abbvie, BMS, Celgene, Eli Lilly, Novartis and Roche.
Figures

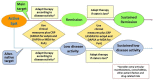
Comment in
-
Spondyloarthritis: Reinforcing 'treat to target' for SpA.Nat Rev Rheumatol. 2017 Sep;13(9):514. doi: 10.1038/nrrheum.2017.122. Epub 2017 Aug 3. Nat Rev Rheumatol. 2017. PMID: 28769113 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

