Calcium and Excitation-Contraction Coupling in the Heart
- PMID: 28684623
- PMCID: PMC5497788
- DOI: 10.1161/CIRCRESAHA.117.310230
Calcium and Excitation-Contraction Coupling in the Heart
Abstract
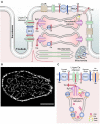
Cardiac contractility is regulated by changes in intracellular Ca concentration ([Ca2+]i). Normal function requires that [Ca2+]i be sufficiently high in systole and low in diastole. Much of the Ca needed for contraction comes from the sarcoplasmic reticulum and is released by the process of calcium-induced calcium release. The factors that regulate and fine-tune the initiation and termination of release are reviewed. The precise control of intracellular Ca cycling depends on the relationships between the various channels and pumps that are involved. We consider 2 aspects: (1) structural coupling: the transporters are organized within the dyad, linking the transverse tubule and sarcoplasmic reticulum and ensuring close proximity of Ca entry to sites of release. (2) Functional coupling: where the fluxes across all membranes must be balanced such that, in the steady state, Ca influx equals Ca efflux on every beat. The remainder of the review considers specific aspects of Ca signaling, including the role of Ca buffers, mitochondria, Ca leak, and regulation of diastolic [Ca2+]i.
Keywords: calcium; cytoplasm; mitochondria; ryanodine receptor calcium release channel; sarcoplasmic reticulum.
© 2017 The Authors.
Figures



References
-
- Allen DG, Blinks JR. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978;273:509–513. - PubMed
-
- Allen DG, Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2 ed. Dordrecht/Boston/London: Kluwer Academic Publishers; 2001.
-
- Bers DM, Kranias EG. Normal and abnormal calcium homeostasis. In: Walsh RA, editor. In: Molecular Mechanisms of Cardiac Hypertrophy and Failure. Oxon: Taylor & Francis; 2005. pp. 199–220.
-
- Robinson P, Griffiths PJ, Watkins H, Redwood CS. Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res. 2007;101:1266–1273. doi: 10.1161/CIRCRESAHA.107.156380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

