Impact of Premature Senescence on Radiosensitivity Measured by High Throughput Cell-Based Assays
- PMID: 28684684
- PMCID: PMC5535951
- DOI: 10.3390/ijms18071460
Impact of Premature Senescence on Radiosensitivity Measured by High Throughput Cell-Based Assays
Abstract
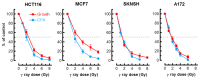
In most p53 wild-type human cell types, radiosensitivity evaluated by the colony formation assay predominantly reflects stress-induced premature senescence (SIPS) and not cell death (Int. J. Mol. Sci. 2017, 18, 928). SIPS is a growth-arrested state in which the cells acquire flattened and enlarged morphology, remain viable, secrete growth-promoting factors, and can give rise to tumor-repopulating progeny. The impact of SIPS on radiosensitivity measured by short-term assays remains largely unknown. We report that in four p53 wild-type human solid tumor-derived cell lines (HCT116, SKNSH, MCF7 and A172): (i) the conventional short-term growth inhibition assay (3 days post-irradiation) generates radiosensitivity data comparable to that measured by the laborious and time-consuming colony formation assay; (ii) radiation dose-response curves obtained by multiwell plate colorimetric/fluorimetric assays are markedly skewed towards radioresistance, presumably reflecting the emergence of highly enlarged, growth-arrested and viable cells; and (iii) radiation exposure (e.g., 8 Gy) does not trigger apoptosis or loss of viability over a period of 3 days post-irradiation. Irrespective of the cell-based assay employed, caution should be exercised to avoid misinterpreting radiosensitivity data in terms of loss of viability and, hence, cell death.
Keywords: CellTitre-Blue; MTT; XTT; apoptosis; colony forming ability; ionizing radiation; p53 signaling; premature senescence; proliferation; viability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Multinucleated Giant Cancer Cells Produced in Response to Ionizing Radiation Retain Viability and Replicate Their Genome.Int J Mol Sci. 2017 Feb 8;18(2):360. doi: 10.3390/ijms18020360. Int J Mol Sci. 2017. PMID: 28208747 Free PMC article.
-
Depletion of securin induces senescence after irradiation and enhances radiosensitivity in human cancer cells regardless of functional p53 expression.Int J Radiat Oncol Biol Phys. 2010 Jun 1;77(2):566-74. doi: 10.1016/j.ijrobp.2009.12.013. Int J Radiat Oncol Biol Phys. 2010. PMID: 20457353
-
Do Multiwell Plate High Throughput Assays Measure Loss of Cell Viability Following Exposure to Genotoxic Agents?Int J Mol Sci. 2017 Aug 2;18(8):1679. doi: 10.3390/ijms18081679. Int J Mol Sci. 2017. PMID: 28767065 Free PMC article.
-
Significance of Wild-Type p53 Signaling in Suppressing Apoptosis in Response to Chemical Genotoxic Agents: Impact on Chemotherapy Outcome.Int J Mol Sci. 2017 Apr 28;18(5):928. doi: 10.3390/ijms18050928. Int J Mol Sci. 2017. PMID: 28452953 Free PMC article. Review.
-
Ionizing radiation-induced responses in human cells with differing TP53 status.Int J Mol Sci. 2013 Nov 13;14(11):22409-35. doi: 10.3390/ijms141122409. Int J Mol Sci. 2013. PMID: 24232458 Free PMC article. Review.
Cited by
-
Carborane Conjugates with Ibuprofen, Fenoprofen and Flurbiprofen: Synthesis, Characterization, COX Inhibition Potential and In Vitro Activity.ChemMedChem. 2025 Jan 2;20(1):e202400018. doi: 10.1002/cmdc.202400018. Epub 2024 Nov 8. ChemMedChem. 2025. PMID: 38844420 Free PMC article.
-
Roles of Polyploid/Multinucleated Giant Cancer Cells in Metastasis and Disease Relapse Following Anticancer Treatment.Cancers (Basel). 2018 Apr 15;10(4):118. doi: 10.3390/cancers10040118. Cancers (Basel). 2018. PMID: 29662021 Free PMC article.
-
Interruptin C, a Radioprotective Agent, Derived from Cyclosorus terminans Protect Normal Breast MCF-10A and Human Keratinocyte HaCaT Cells against Radiation-Induced Damage.Molecules. 2022 May 20;27(10):3298. doi: 10.3390/molecules27103298. Molecules. 2022. PMID: 35630775 Free PMC article.
-
Apoptotic Effects of Xanthium strumarium via PI3K/AKT/mTOR Pathway in Hepatocellular Carcinoma.Evid Based Complement Alternat Med. 2019 Nov 7;2019:2176701. doi: 10.1155/2019/2176701. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31885640 Free PMC article.
-
Chromatin and the Cellular Response to Particle Radiation-Induced Oxidative and Clustered DNA Damage.Front Cell Dev Biol. 2022 Jul 13;10:910440. doi: 10.3389/fcell.2022.910440. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35912116 Free PMC article. Review.
References
-
- Murray D., Mirzayans R. Role of therapy-induced cellular senescence in tumor cells and its modification in radiotherapy; the good, the bad and the ugly. J. Nucl. Med. Radiat. Ther. 2013;S6:018.
-
- Chang B.D., Broude E.V., Dokmanovic M., Zhu H., Ruth A., Xuan Y., Kandel E.S., Lausch E., Christov K., Roninson I.B. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59:3761–3767. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

