Differentiating Staphylococcus aureus from Escherichia coli mastitis: S. aureus triggers unbalanced immune-dampening and host cell invasion immediately after udder infection
- PMID: 28684793
- PMCID: PMC5500526
- DOI: 10.1038/s41598-017-05107-4
Differentiating Staphylococcus aureus from Escherichia coli mastitis: S. aureus triggers unbalanced immune-dampening and host cell invasion immediately after udder infection
Abstract
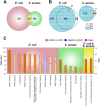
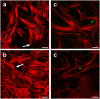
The etiology determines quality and extent of the immune response after udder infection (mastitis). Infections with Gram negative bacteria (e.g. Escherichia coli) will quickly elicit strong inflammation of the udder, fully activate its immune defence via pathogen receptor driven activation of IκB/NF-κB signaling. This often eradicates the pathogen. In contrast, Gram-positive bacteria (e.g. Staphylococcus aureus) will slowly elicit a much weaker inflammation and immune response, frequently resulting in chronic infections. However, it was unclear which immune regulatory pathways are specifically triggered by S. aureus causing this partial immune subversion. We therefore compared in first lactating cows the earliest (1-3 h) udder responses against infection with mastitis causing pathogens of either species. Global transcriptome profiling, bioinformatics analysis and experimental validation of key aspects revealed as S. aureus infection specific features the (i) failure to activating IκB/NF-κB signaling; (ii) activation of the wnt/β-catenin cascade resulting in active suppression of NF-κB signaling and (iii) rearrangement of the actin-cytoskeleton through modulating Rho GTPase regulated pathways. This facilitates invasion of pathogens into host cells. Hence, S. aureus mastitis is characterized by eliciting unbalanced immune suppression rather than inflammation and invasion of S. aureus into the epithelial cells of the host causing sustained infection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

