A retrospective study to assess clinical characteristics and time to initiation of open-triple therapy among patients with chronic obstructive pulmonary disease, newly established on long-acting mono- or combination therapy
- PMID: 28684905
- PMCID: PMC5485896
- DOI: 10.2147/COPD.S129007
A retrospective study to assess clinical characteristics and time to initiation of open-triple therapy among patients with chronic obstructive pulmonary disease, newly established on long-acting mono- or combination therapy
Abstract
Introduction: An incremental approach using open-triple therapy may improve outcomes in patients with chronic obstructive pulmonary disease (COPD). However, there is little sufficient, real-world evidence available identifying time to open-triple initiation.
Methods: This retrospective study of patients with COPD, newly initiated on long-acting muscarinic antagonist (LAMA) monotherapy or inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) combination therapy, assessed baseline demographics, clinical characteristics, and exacerbations during 12 months prior to first LAMA or ICS/LABA use. Time to initiation of open-triple therapy was assessed for 12 months post-index date. Post hoc analyses were performed to assess the subsets of patients with pulmonary-function test (PFT) information and patients with and without comorbid asthma.
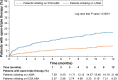
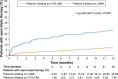
Results: Demographics and clinical characteristics were similar between cohorts in the pre-specified and post hoc analyses. In total, 283 (19.3%) and 160 (10.9%) patients had moderate and severe exacerbations at baseline, respectively, in the LAMA cohort, compared with 482 (21.3%) and 289 (12.8%) patients in the ICS/LABA cohort. Significantly more patients initiated open-triple therapy in the LAMA cohort compared with the ICS/LABA cohort (226 [15.4%] versus 174 [7.7%]; P<0.001); results were similar in the post hoc analyses. Mean (standard deviation) time to open-triple therapy was 79.8 (89.0) days in the LAMA cohort and 122.9 (105.4) days in the ICS/LABA cohort (P<0.001). This trend was also observed in the post hoc analyses, though the difference between cohorts was nonsignificant in the subset of patients with PFT information.
Discussion: In this population, patients with COPD are more likely to initiate open-triple therapy following LAMA therapy, compared with ICS/LABA therapy. Further research is required to identify factors associated with the need for treatment augmentation among patients with COPD.
Keywords: COPD; inhaled corticosteroids; long-acting muscarinic antagonists; long-acting β-agonists; open-triple therapy.
Conflict of interest statement
Disclosures DM and MHR were employed by Lovelace Clinic Foundation at the time this study was conducted. Besides the funding Lovelace Clinic Foundation received to conduct this study, both have received grant funding from Boehringer Ingelheim Pharmaceuticals Inc., GSK, EndoPharmaceuticals, and Pfizer Inc. to conduct other COPD-related research. DM has also received grant funding from Sunovian Pharmaceuticals for other research unrelated to this project. SRS and DS are employees of Reliant Medical Group, who received research funding from GSK to conduct the study. DP, FL, MSD, and PL are employees of Groupe d’analyse/Analysis Group, a consulting company that received research funds from GSK to conduct the study. Groupe d’analyse/Analysis Group has also received research funds from GSK, Novartis, Janssen Pharmaceutical Affairs, LLC, Bayer, and other pharmaceutical companies to conduct other respiratory disease studies. JP was an employee of GSK at the time of study conduct and owned stocks and shares in GSK. He is currently employed by, and owns stocks and shares in, Amgen. The authors report no other conflicts of interest in this work.
Figures


References
-
- GOLD Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [updated 2017] [Accessed January 25, 2017]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-managem....
-
- Cheyne L, Irvin-Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;9:CD009552. - PubMed
-
- Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

