α-bisabolol-loaded lipid-core nanocapsules reduce lipopolysaccharide-induced pulmonary inflammation in mice
- PMID: 28684908
- PMCID: PMC5484570
- DOI: 10.2147/IJN.S130798
α-bisabolol-loaded lipid-core nanocapsules reduce lipopolysaccharide-induced pulmonary inflammation in mice
Abstract
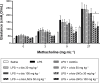
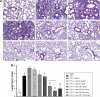
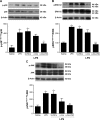
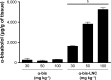
Acute respiratory distress syndrome (ARDS) is a severe clinical condition of respiratory failure due to an intense inflammatory response with different etiologies. Despite all efforts, therapy remains limited, and ARDS is still associated with high mortality and morbidity. Plants can provide a vast source of active natural products for the discovery of new drugs. α-bisabolol (α-bis), a constituent of the essential oil from chamomile, has elicited pharmacological interest. However, the molecule has some limitations to its biological application. This study was conducted to develop a drug delivery system using lipid-core nanocapsules (LNCs) to improve the anti-inflammatory effects of orally administered α-bis. α-bis-loaded LNCs (α-bis-LNCs) were prepared by interfacial deposition of poly(ε-caprolactone) and orally administered in a mouse model of ARDS triggered by an intranasal administration of lipopolysaccharide (LPS). We found that α-bis-LNCs (30, 50, and 100 mg kg-1) significantly reduced airway hyperreactivity (AHR), neutrophil infiltration, myeloperoxidase activity, chemokine levels (KC and MIP-2), and tissue lung injury 18 hours after the LPS challenge. By contrast, free α-bis failed to modify AHR and neutrophil accumulation in the bronchoalveolar lavage effluent and lung parenchyma and inhibited elevation in the myeloperoxidase and MIP-2 levels only at the highest dose. Furthermore, only α-bis-LNCs reduced LPS-induced changes in mitogen-activated protein kinase signaling, as observed by a significant reduction in phosphorylation levels of ERK1/2, JNK, and p38 proteins. Taken together, our results clearly show that by using LNCs, α-bis was able to decrease LPS-induced inflammation. These findings may be explained by the robust increase of α-bis concentration in the lung tissue that was achieved by the LNCs. Altogether, these results indicate that α-bis-LNCs should further be investigated as a potential alternative for the treatment of ARDS.
Keywords: acute respiratory distress syndrome; anti-inflammatory effects; drug delivery; nanotechnology; pulmonary inflammation; α-bisabolol.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Orally delivered resveratrol-loaded lipid-core nanocapsules ameliorate LPS-induced acute lung injury via the ERK and PI3K/Akt pathways.Int J Nanomedicine. 2019 Jul 12;14:5215-5228. doi: 10.2147/IJN.S200666. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31371957 Free PMC article.
-
Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice.Int Immunopharmacol. 2009 Feb;9(2):194-200. doi: 10.1016/j.intimp.2008.11.004. Epub 2008 Dec 9. Int Immunopharmacol. 2009. PMID: 19071231
-
Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen-activated protein kinase.J Immunol. 2002 Nov 1;169(9):5260-9. doi: 10.4049/jimmunol.169.9.5260. J Immunol. 2002. PMID: 12391245
-
Nucleic-Acid Delivery Using Lipid Nanocapsules.Curr Pharm Biotechnol. 2016;17(8):723-7. doi: 10.2174/1389201017666160401145206. Curr Pharm Biotechnol. 2016. PMID: 27033510 Review.
-
Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation.Eur Respir J. 2020 Jul 30;56(1):1901298. doi: 10.1183/13993003.01298-2019. Print 2020 Jul. Eur Respir J. 2020. PMID: 32299854 Review.
Cited by
-
Chemical Characterization and Chemotaxonomic Significance of Essential Oil Constituents of Matricaria aurea Grown in Two Different Agro-Climatic Conditions.Plants (Basel). 2023 Oct 12;12(20):3553. doi: 10.3390/plants12203553. Plants (Basel). 2023. PMID: 37896017 Free PMC article.
-
Nanotherapeutics in the treatment of acute respiratory distress syndrome.Life Sci. 2021 Jul 1;276:119428. doi: 10.1016/j.lfs.2021.119428. Epub 2021 Mar 27. Life Sci. 2021. PMID: 33785346 Free PMC article. Review.
-
Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol.Nutrients. 2022 Mar 25;14(7):1370. doi: 10.3390/nu14071370. Nutrients. 2022. PMID: 35405982 Free PMC article. Review.
-
α-Bisabolol Mitigates Colon Inflammation by Stimulating Colon PPAR-γ Transcription Factor: In Vivo and In Vitro Study.PPAR Res. 2022 Apr 13;2022:5498115. doi: 10.1155/2022/5498115. eCollection 2022. PPAR Res. 2022. PMID: 35465355 Free PMC article.
-
Oleic acid-based nanosystems for mitigating acute respiratory distress syndrome in mice through neutrophil suppression: how the particulate size affects therapeutic efficiency.J Nanobiotechnology. 2020 Jan 31;18(1):25. doi: 10.1186/s12951-020-0583-y. J Nanobiotechnology. 2020. PMID: 32005196 Free PMC article.
References
-
- Arroliga AC, Ghamra ZW, Perez Trepichio A, et al. Incidence of ARDS in an adult population of northeast Ohio. Chest. 2002;121(6):1972–1976. - PubMed
-
- Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1159–1164. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2004;342(18):1334–1349. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

