Molecular docking, PASS analysis, bioactivity score prediction, synthesis, characterization and biological activity evaluation of a functionalized 2-butanone thiosemicarbazone ligand and its complexes
- PMID: 28684996
- PMCID: PMC5480261
- DOI: 10.1007/s12154-017-0167-y
Molecular docking, PASS analysis, bioactivity score prediction, synthesis, characterization and biological activity evaluation of a functionalized 2-butanone thiosemicarbazone ligand and its complexes
Abstract
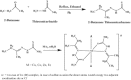
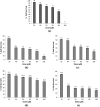
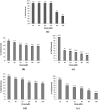
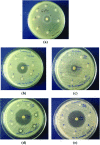
2-Butanone thiosemicarbazone ligand was prepared by condensation reaction between thiosemicarbazide and butanone. The ligand was characterized by 1H NMR, 13C NMR, FT-IR, mass spectrometry and UV spectroscopic studies. Docking studies were performed to study inhibitory action against topoisomerase II (Topo II) and ribonucleoside diphosphate reductase (RR) enzymes. Inhibition constants (Ki ) of the ligand were 437.87 and 327.4 μM for the two enzymes, respectively. The ligand was tested for its potential anticancer activity against two cancer cell lines MDA-MB-231 and A549 using MTT assay and was found to exhibit good activity at higher doses with an IC50 = 80 μM against human breast cancer cell line MDA-MB-231. On the other hand, no significant activity was obtained against the lung carcinoma cell line A549. Antibacterial activity of the ligand was tested against Staphylococcus aureus and E. coli using the disc diffusion method. Ligand did not exhibit any significant antibacterial activity. Four complexes of Co(III), Fe(II), Cu(II), and Zn(II) were prepared with the ligand and characterized by various spectroscopic studies. Low molar conductance values were obtained for all complexes displaying non-electrolyte nature except in Co(III) complex. As expected, complexation with metal ions significantly increased the cytotoxicity of the ligand against the tested cell lines viz. IC50 values of <20 μM for Co, Fe, and Zn complexes and approx. 80 μM against MDA cells versus IC50 value of <20 μM for Co and Cu complexes and that of 30 and 50 μM for Fe and Zn complexes, respectively, against A549 cells. The Cu complex was found to be active against E. coli and S. aureus with MIC values in the range of 6-10 mg/mL. Other than Cu, only Co complex was found to possess antibacterial activity with MIC values of 5-10 mg/mL when tested against S. aureus. Bioactivity score and Prediction of Activity Spectra for Substances (PASS) analysis also depicted the drug-like nature of ligand and complexes.
Keywords: Antibacterial; Anticancer; Docking; PASS; Ribonucleotide reductase; Thiosemicarbazone; Topoisomerase II.
Conflict of interest statement
Conflict of interest
The authors declare that they have no competing interests.
Figures


Similar articles
-
Synthesis, characterization, computational studies and biological activity evaluation of Cu, Fe, Co and Zn complexes with 2-butanone thiosemicarbazone and 1,10-phenanthroline ligands as anticancer and antibacterial agents.EXCLI J. 2018 Mar 29;17:331-348. doi: 10.17179/excli2017-984. eCollection 2018. EXCLI J. 2018. PMID: 29743867 Free PMC article.
-
Mixed Ligand-metal Complexes of 2-(butan-2-ylidene) Hydrazinecarbothioamide- Synthesis, Characterization, Computer-Aided Drug Character Evaluation and in vitro Biological Activity Assessment.Curr Comput Aided Drug Des. 2021;17(1):107-122. doi: 10.2174/1573409915666190926122103. Curr Comput Aided Drug Des. 2021. PMID: 31556860
-
Computer-aided drug design and virtual screening of targeted combinatorial libraries of mixed-ligand transition metal complexes of 2-butanone thiosemicarbazone.Comput Biol Chem. 2018 Aug;75:178-195. doi: 10.1016/j.compbiolchem.2018.05.008. Epub 2018 May 8. Comput Biol Chem. 2018. PMID: 29883916
-
Ligational behaviour of lomefloxacin drug towards Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Th(IV) and UO(2)(VI) ions: synthesis, structural characterization and biological activity studies.Spectrochim Acta A Mol Biomol Spectrosc. 2011 Nov;82(1):8-19. doi: 10.1016/j.saa.2011.05.089. Epub 2011 Jun 1. Spectrochim Acta A Mol Biomol Spectrosc. 2011. PMID: 21855402
-
Recent progress in thiocarbazone metal complexes for cancer therapy via mitochondrial signalling pathway.Front Chem. 2024 May 30;12:1424022. doi: 10.3389/fchem.2024.1424022. eCollection 2024. Front Chem. 2024. PMID: 38873408 Free PMC article. Review.
Cited by
-
Essential oil from Cymbopogon citratus exhibits "anti-aspergillosis" potential: in-silico molecular docking and in vitro studies.Bull Natl Res Cent. 2022;46(1):23. doi: 10.1186/s42269-022-00711-5. Epub 2022 Jan 29. Bull Natl Res Cent. 2022. PMID: 35125860 Free PMC article.
-
Essential Oil Derived from Underutilized Plants Cymbopogon khasianus Poses Diverse Biological Activities against "Aspergillosis" and "Mucormycosis".Russ Agric Sci. 2023;49(2):172-183. doi: 10.3103/S106836742302012X. Epub 2023 May 17. Russ Agric Sci. 2023. PMID: 37220552 Free PMC article.
-
In vitro cellular and molecular plus in silico studies of a substituted bipyridine-coordinated Zn(II) ion: cytotoxicity, ROS-induced apoptosis, anti-metastasis, and BAX/BCL2 genes expression.J Biol Inorg Chem. 2025 Aug;30(4-5):359-379. doi: 10.1007/s00775-025-02114-z. Epub 2025 Apr 20. J Biol Inorg Chem. 2025. PMID: 40253669
-
Computational exploration of novel ROCK2 inhibitors for cardiovascular disease management; insights from high-throughput virtual screening, molecular docking, DFT and MD simulation.PLoS One. 2023 Nov 16;18(11):e0294511. doi: 10.1371/journal.pone.0294511. eCollection 2023. PLoS One. 2023. PMID: 37972144 Free PMC article.
-
An Evaluation of the Potential of Essential Oils against SARS-CoV-2 from In Silico Studies through the Systematic Review Using a Chemometric Approach.Pharmaceuticals (Basel). 2021 Nov 10;14(11):1138. doi: 10.3390/ph14111138. Pharmaceuticals (Basel). 2021. PMID: 34832920 Free PMC article. Review.
References
-
- Al-Amiery AA, Mohammed A, Ibrahim H, Abbas A. J Biotechnol Res Cent. 2010;4:5.
-
- Barnum W. J Inorg Nucl Chem. 1961;21:221. doi: 10.1016/0022-1902(61)80297-2. - DOI
-
- Beraldo H, Gambino D. Med Chem. 2004;4:31. - PubMed
-
- Bjelogrlic S, Todorovic T, Bacchi A, Zec M, Sladic D, Srdic-Rajic T. J Inorg Biochem. 2010;104(673):12–1339. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

