Combination of irinotecan and platinum for platinum-resistant or refractory recurrent ovarian cancers: A preliminary case series
- PMID: 28685075
- PMCID: PMC5492769
- DOI: 10.3892/mco.2017.1258
Combination of irinotecan and platinum for platinum-resistant or refractory recurrent ovarian cancers: A preliminary case series
Abstract
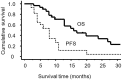
Non-platinum single agents are usually used for patients with platinum-resistant recurrent ovarian cancers (ROC). However, the efficacy of these drugs is limited. The aim of the present study was to evaluate the efficacy and adverse events (AE) of combination therapy with irinotecan and platinum (CPT-Pt) for ROC. A total of 28 platinum-resistant or refractory patients with ROC treated with CPT-Pt at the National Defense Medical College Hospital institution between 2002 and 2012 were identified. All patients received taxane and carboplatin (TC) as a first-line treatment and relapsed within 6 months after completion of TC, or progressed during TC therapy. The median age was 59 years (range, 16-78), and median number of CPT-Pt therapy cycles was 5.5 (range, 2-16). The overall response rate was 14%, with a complete response (CR) in 2 patients and partial response (PR) in 2 patients. Stable disease (SD) for >3 months was observed in 15 patients (54%), resulting in a clinical benefit rate (CBR = CR + PR + SD) of 68%. The median progression-free survival and overall survival were 8 and 15 months, respectively. Fifteen cases (68%) developed grade 3/4 hematological AE and 3 cases (11%) developed non-hematological grade 3/4 AE, which were resolved by conservative management or dose reduction. Platinum re-treatment with irinotecan for platinum refractory or resistant ROC may be a candidate in such clinical settings.
Keywords: irinotecan; platinum; platinum-resistant; recurrent ovarian cancer.
Figures
References
-
- Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, Wang Y, Scribner DR, Jr, Marciniack M, Naumann RW, Secord AA. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–2818. doi: 10.1200/JCO.2006.09.6735. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous