Effects of Metformin and Furosemide on Rosuvastatin Pharmacokinetics in Healthy Volunteers: Implications for Their Use as Probe Drugs in a Transporter Cocktail
- PMID: 28685495
- PMCID: PMC5794840
- DOI: 10.1007/s13318-017-0427-9
Effects of Metformin and Furosemide on Rosuvastatin Pharmacokinetics in Healthy Volunteers: Implications for Their Use as Probe Drugs in a Transporter Cocktail
Abstract
Background: In a recently described probe drug cocktail for clinically relevant drug transporters containing digoxin, furosemide, metformin and rosuvastatin, mutual interactions were essentially absent except for increases in the systemic exposure of rosuvastatin. To optimize the cocktail, we further examined the dose dependence of the effects of metformin and furosemide on rosuvastatin pharmacokinetics.
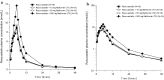
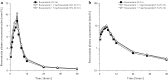
Methods: This was a randomized, open label, single center, six-treatment, six-period, six-sequence crossover trial. Eighteen healthy male subjects received 10 mg rosuvastatin as reference treatment and, as test treatments, 10 mg rosuvastatin combined with 10, 50 or 500 mg metformin (T1, T2 and T3) or with 1 or 5 mg furosemide (T4 and T5). Primary pharmacokinetic endpoints were rosuvastatin C max (maximum plasma concentration) and AUC0-tz (area under the plasma concentration-time curve from time zero to the last quantifiable concentration).
Results: The relative bioavailability of rosuvastatin was essentially unchanged when administered with metformin in T1 and T2, but in T3 it increased to 152% for AUC0-tz (90% CI 135-171%) and 154% for C max (90% CI 132-180%). Coadministration with furosemide did not change rosuvastatin relative bioavailability in T4, but in T5 it increased slightly to 116% for AUC0-tz (90% CI 102-132%) and 118% for C max (90% CI 98-142%).
Conclusion: The increased systemic exposure of rosuvastatin when administered as part of the proposed transporter cocktail is most likely attributable to metformin and only to a minor degree to furosemide. Reduction of the doses of metformin and furosemide is expected to eliminate the previously described interaction. EudraCT no. 2015-003052-46, ClinicalTrials.gov identifier NCT02574845.
Conflict of interest statement
Funding
The study was funded by Boehringer Ingelheim Pharma GmbH & Co. KG, the Sponsor of the trial.
Conflicts of interest
Kathrin Hohl was contracted by Boehringer Ingelheim as an external statistician. All other authors are employees of Boehringer Ingelheim.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The clinical trial protocol was approved by the Ethics Commission of the State Chamber of Physicians of Baden-Württemberg, Stuttgart, Germany, and the Federal Institute for Drugs and Medicinal Products (BfArM), Bonn, Germany.
Informed consent
Written informed consent was obtained from all individual participants included in this trial.
Figures
Similar articles
-
Pharmacokinetics of Rosuvastatin: A Systematic Review of Randomised Controlled Trials in Healthy Adults.Clin Pharmacokinet. 2021 Feb;60(2):165-175. doi: 10.1007/s40262-020-00978-9. Epub 2021 Jan 11. Clin Pharmacokinet. 2021. PMID: 33428168
-
Optimization of a drug transporter probe cocktail: potential screening tool for transporter-mediated drug-drug interactions.Br J Clin Pharmacol. 2018 Sep;84(9):1941-1949. doi: 10.1111/bcp.13609. Epub 2018 Jun 21. Br J Clin Pharmacol. 2018. PMID: 29665130 Free PMC article. Clinical Trial.
-
The Effect of BI 730357 (Retinoic Acid-Related Orphan Receptor Gamma t Antagonist, Bevurogant) on the Pharmacokinetics of a Transporter Probe Cocktail, Including Digoxin, Furosemide, Metformin, and Rosuvastatin: An Open-Label, Non-randomized, 2-Period Fixed-Sequence Trial in Healthy Subjects.Clin Pharmacol Drug Dev. 2024 Feb;13(2):197-207. doi: 10.1002/cpdd.1344. Epub 2023 Nov 13. Clin Pharmacol Drug Dev. 2024. PMID: 37960990
-
Pharmacokinetic Evaluation of a Drug Transporter Cocktail Consisting of Digoxin, Furosemide, Metformin, and Rosuvastatin.Clin Pharmacol Ther. 2016 Sep;100(3):259-67. doi: 10.1002/cpt.406. Epub 2016 Jul 29. Clin Pharmacol Ther. 2016. PMID: 27256812 Free PMC article. Clinical Trial.
-
Furosemide pharmacokinetics and pharmacodynamics in health and disease--an update.J Pharmacokinet Biopharm. 1989 Feb;17(1):1-46. doi: 10.1007/BF01059086. J Pharmacokinet Biopharm. 1989. PMID: 2654356 Review.
Cited by
-
Pharmacokinetics of Rosuvastatin: A Systematic Review of Randomised Controlled Trials in Healthy Adults.Clin Pharmacokinet. 2021 Feb;60(2):165-175. doi: 10.1007/s40262-020-00978-9. Epub 2021 Jan 11. Clin Pharmacokinet. 2021. PMID: 33428168
-
Optimization of a drug transporter probe cocktail: potential screening tool for transporter-mediated drug-drug interactions.Br J Clin Pharmacol. 2018 Sep;84(9):1941-1949. doi: 10.1111/bcp.13609. Epub 2018 Jun 21. Br J Clin Pharmacol. 2018. PMID: 29665130 Free PMC article. Clinical Trial.
-
Statins for the prevention of proliferative vitreoretinopathy: cellular responses in cultured cells and clinical statin concentrations in the vitreous.Sci Rep. 2021 Jan 13;11(1):980. doi: 10.1038/s41598-020-80127-1. Sci Rep. 2021. PMID: 33441813 Free PMC article.
-
Validation of a Drug Transporter Probe Cocktail Using the Prototypical Inhibitors Rifampin, Probenecid, Verapamil, and Cimetidine.Clin Pharmacokinet. 2020 Dec;59(12):1627-1639. doi: 10.1007/s40262-020-00907-w. Clin Pharmacokinet. 2020. PMID: 32504272 Free PMC article. Clinical Trial.
-
Metformin and statin use associate with plasma protein N-glycosylation in people with type 2 diabetes.BMJ Open Diabetes Res Care. 2020 Jul;8(1):e001230. doi: 10.1136/bmjdrc-2020-001230. BMJ Open Diabetes Res Care. 2020. PMID: 32616483 Free PMC article.
References
-
- EMA-CHMP. Guideline on the investigation of drug interactions: final (CPMP/EWP/560/95/Rev. 1 corr. 2). 2012 Jun. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 13 March 2017.
-
- US-FDA. Guidance for industry: drug interaction studies - study design, data analysis, implications for dosing, and labeling recommendations (draft guidance). 2012 Feb. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Accessed 13 March 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

